December 27, 2022, Deadline for Mandatory Rx Data Collection Reporting

As group health plan sponsors, employers are responsible for ensuring compliance with the prescription drug data collection (RxDC) reporting requirements

Several New Group Health Plan Reporting Deadlines Are Approaching – Check Out Our CAA/Price Transparency Checklist

Federal Register :: Medicare Program; Contract Year 2024 Policy and Technical Changes to the Medicare Advantage Program, Medicare Prescription Drug Benefit Program, Medicare Cost Plan Program, Medicare Parts A, B, C, and

Second Prescription Drug (RxDC) Report for Pharmacy Transparency in Health Plans Is Due by June 1, 2023

Federal Register :: Medicare Program; Contract Year 2024 Policy and Technical Changes to the Medicare Advantage Program, Medicare Prescription Drug Benefit Program, Medicare Cost Plan Program, Medicare Parts A, B, C, and
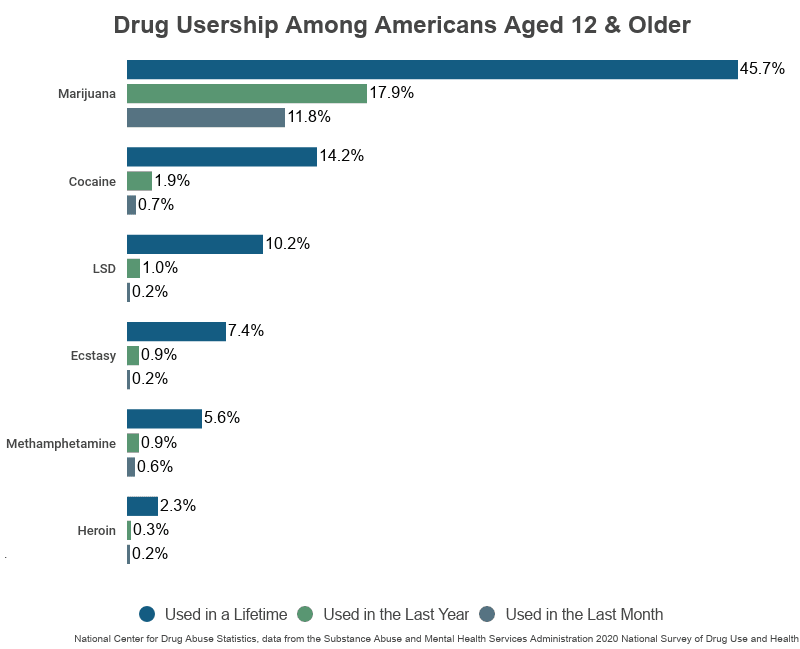
NCDAS: Substance Abuse and Addiction Statistics [2023]

Compliance Alert: Agencies Extend RxDC Reporting Deadline to January 31, 2023

Prescription Data Collection Report Coming Soon - EPIC Brokers

Misleading Ads Fueled Rapid Growth of Online Mental Health Companies - WSJ

Federal Register :: Medicare Program; Contract Year 2024 Policy and Technical Changes to the Medicare Advantage Program, Medicare Prescription Drug Benefit Program, Medicare Cost Plan Program, Medicare Parts A, B, C, and

Prescription Drug Reporting Due by December 27, 2022

Prescription Drug Reporting Due by Dec. 27, 2022 - Seubert

Prescription Drug Reporting Due by Dec. 27, 2022

Index of /wp-content/uploads/2022/10

RxDC Reporting Will Bring Changes . . . Here's What to Know Now - Word on Benefits







