2024-2025 Regulatory Agenda Has Been Approved by the Brazilian FDA
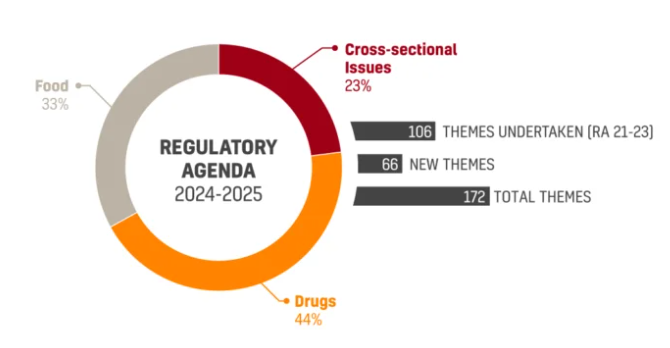
ANVISA’s new Regulatory Agenda 2024-2025 (RA 24/25) was approved and published on the Official Gazette on December 18th. The Joint Ordinance No. 1…

Simply Buckhead January/February 2024 by Simply Buckhead - Issuu

2024-2025 Regulatory Agenda Has Been Approved by the Brazilian FDA - Lexology

How to Prepare for and Make the Most Out of your FDA Pre-Submission: Leverage This Under-Utilized Tool to Help De-Risk your 510(k)

How to Prepare for and Make the Most Out of your FDA Pre-Submission: Leverage This Under-Utilized Tool to Help De-Risk your 510(k)

How to Prepare for and Make the Most Out of your FDA Pre-Submission: Leverage This Under-Utilized Tool to Help De-Risk your 510(k)

Forecasting the Future of Stroke in the United States

News

Federal Register :: Medicare Program; Contract Year 2025 Policy and Technical Changes to the Medicare Advantage Program, Medicare Prescription Drug Benefit Program, Medicare Cost Plan Program, and Programs of All-Inclusive Care for

FDA's BsUFA III Features

Inline XBRL Viewer

Sports betting is now regulated in Brazil