Xpert® Xpress SARS-CoV-2 - FDA Emergency Use Authorization
.png)
Xpert® Xpress SARS-CoV-2/Flu/RSV received Emergency Use Authorization from the US FDA to support the global fight against COVID-19, with rapid detection of the current coronavirus SARS-CoV-2.

Cepheid Receives Emergency Use Authorization For SARS-CoV-2, Flu A, Flu B, and RSV Combination Test
.png)
Xpert® Xpress SARS-CoV-2 - FDA Emergency Use Authorization

FDA grants EUA to Cepheid's rapid Covid-19/Flu/RSV diagnostic test

Diagnostic accuracy of the Cepheid Xpert Xpress and the Abbott ID NOW assay for rapid detection of SARS‐CoV‐2: A systematic review and meta‐analysis - Lee - 2021 - Journal of Medical Virology

FDA authorized molecular point-of-care SARS-CoV-2 tests: A critical review on principles, systems and clinical performances - ScienceDirect
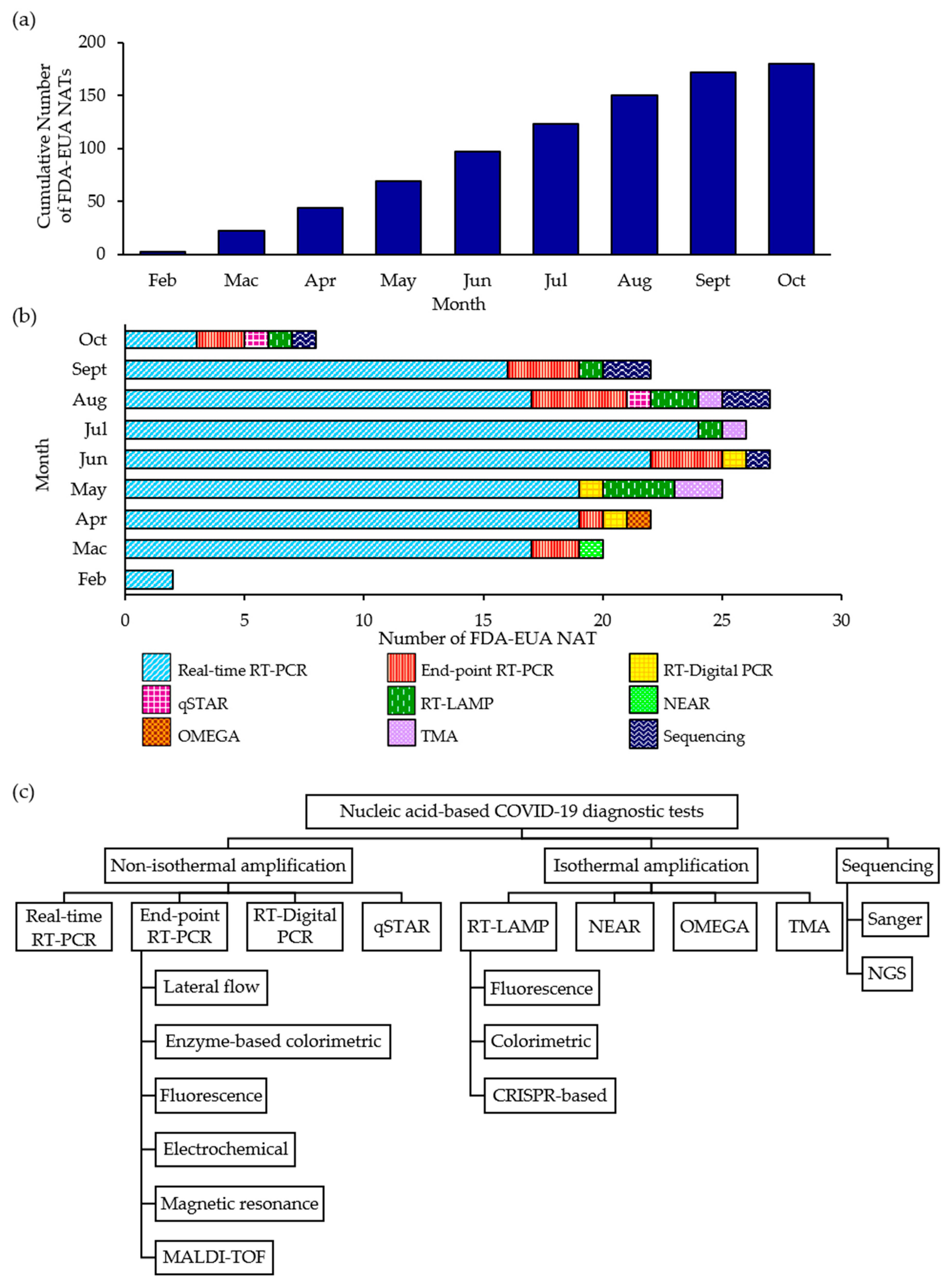
Diagnostics, Free Full-Text

Multicenter Evaluation of the Cepheid Xpert Xpress SARS-CoV-2 Test

FDA approves new coronavirus test that can have 'results within hours' instead of days

Four-In-One-Test for Respiratory Virus Season

Coronavirus COVID-19
/image%2F1237073%2F20210907%2Fob_55d8e5_crayon-couleur-nounoucoindespetits-ove.jpg)




:max_bytes(150000):strip_icc()/Why-You-Should-Be-Very-Careful-With-the-Liquid-in-Your-COVID-19-Home-Test-GettyImages-1352837936-2000-13c2f1c58c1547f5836c9c56ac92ebf2.jpg)