Avoid Launch Delays By Planning For An FDA-Required REMS Risk
lt;p>Picture this: The FDA accepts a manufacturer's NDA, and the manufacturer plans for its impending launch. But shortly before the anticipated approval, the FDA notifies the manufacturer that a Risk Evaluation and Mitigation Strategy (REMS) program is required to market the product. Now what?</p>

Abby Proch on LinkedIn: Avoid Launch Delays By Planning For An FDA-Required REMS

Avoid Launch Delays By Planning For An FDA-Required REMS Risk Evaluation and Mitigation Strategy

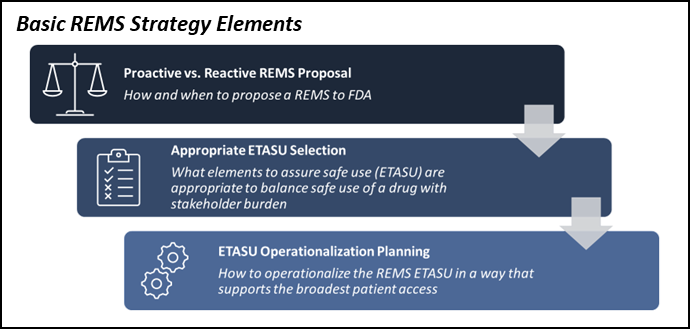
Improving Risk Evaluation and Mitigation Strategy

Responding to the opioid crisis in North America and beyond: recommendations of the Stanford–Lancet Commission - The Lancet

Chimeric Antigen Receptor T-Cell Therapies: Barriers and Solutions to Access

Figure A4. Text extracted from REMS materials to illustrate content

FDA Risk Evaluation and Mitigation Strategies (REMS): Description and Effect on Generic Drug Development

PDF) Don't sell out safety: A call to preserve risk evaluation and mitigation strategies to reduce harm to patients and the public in the U.S.

A Look Back at Risk Evaluation and Mitigation Strategies at the Food and Drug Administration in 2020: Year in Review - Food and Drug Law Institute (FDLI)
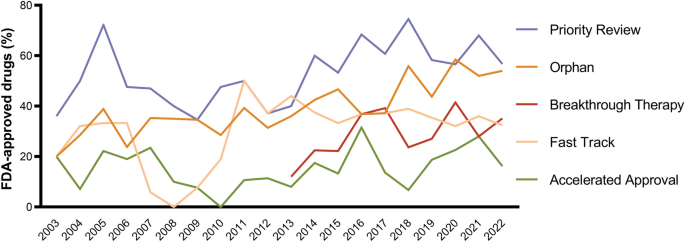
Special FDA designations for drug development: orphan, fast track, accelerated approval, priority review, and breakthrough therapy

Drug Safety and the Cost of Monitoring: The Role of REMS in Risk Management - Mark Slomiany, Rema Bitar, Sarah Kruse, Sarah Jeffers, Kenneth Berkowitz, Mahmud Hassan, 2015
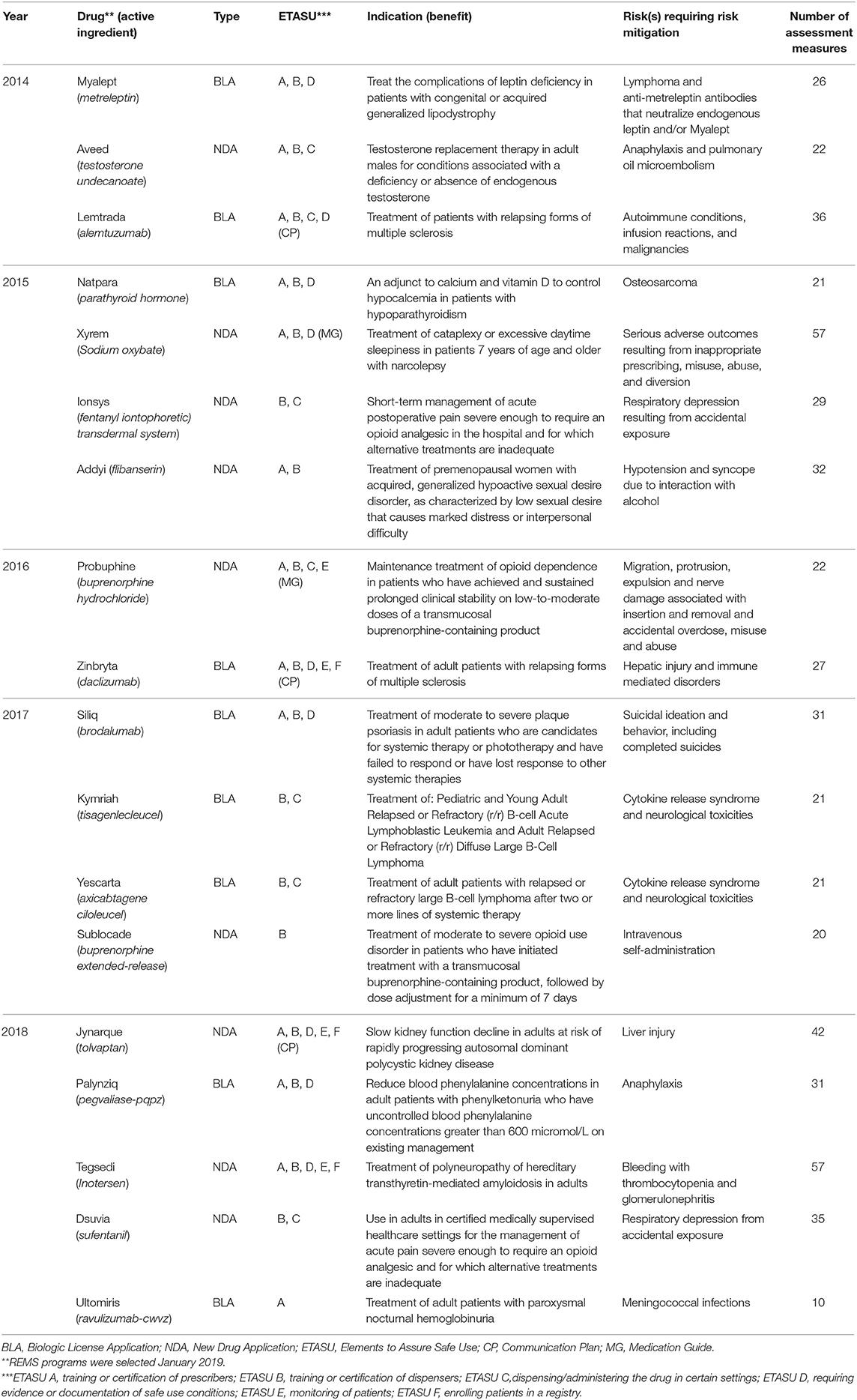
Frontiers Adaptation for Regulatory Application: A Content Analysis of FDA Risk Evaluation and Mitigation Strategies Assessment Plans (2014–2018) Using RE-AIM

Understanding Risk Evaluation and Mitigation Strategy (REMS)