Explain the structure of Graphite.

Click here:point_up_2:to get an answer to your question :writing_hand:explain the structure of graphite
Click here👆to get an answer to your question ✍️ Explain the structure of Graphite-
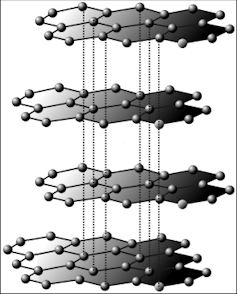
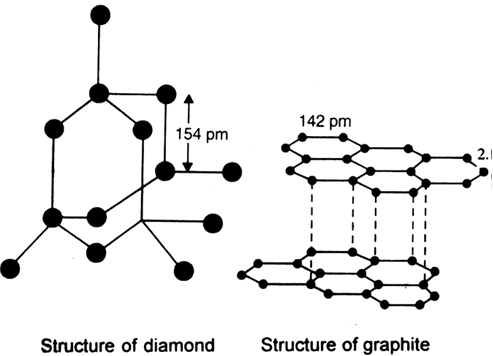
In Graphite each carbon atoms is united with three surrounding carbon atom through covalent bond and forms a sheet-like structure- These sheets or layers are stacked one above the other to form three dimensional structure-Each layer is made up of hexagons there is no covalent bonding between the layers- These layers are held together by weak Vander Wall-s physical forces- Hence these layers can slide over one another

Graphite, another allotrops of carbon

Graphite, Properties, Uses, Structure
Harder than diamond, stronger than steel, super conductor … graphene's unreal

Hybridization of Graphite - Hybridization of Carbon in Graphite

Which of the following structure is similar to graphite? - India Site

Graphite, Properties, Uses, Structure

Draw a well labelled diagram of diamond and graphite and explain their difference.
What are allotropes? Sketch the structure of two allotropes of c
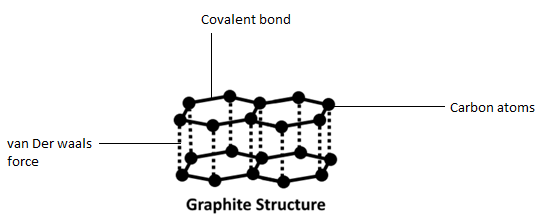
a) What is graphite? What substance is graphite made?(b) Describe the structure of graphite with the help of a labeled diagram.(c) Why is graphite a good conductor of electricity but diamond is









