Graphite, Properties, Uses, Structure

Graphite, mineral consisting of carbon. Graphite has a layered structure that consists of rings of six carbon atoms arranged in widely spaced horizontal sheets. Graphite thus crystallizes in the hexagonal system, in contrast to the same element crystallizing in the octahedral or tetrahedral system

Draw the structure of graphite and diamond. Explain how the structures are responsible for the difference in their physical properties.

Discover Graphite Properties, Structure, Reserves, 10 Uses [PDF] – Design

Graphite is carbon? Carbon graphite properties, use, structure, and diamond - DanCrabon
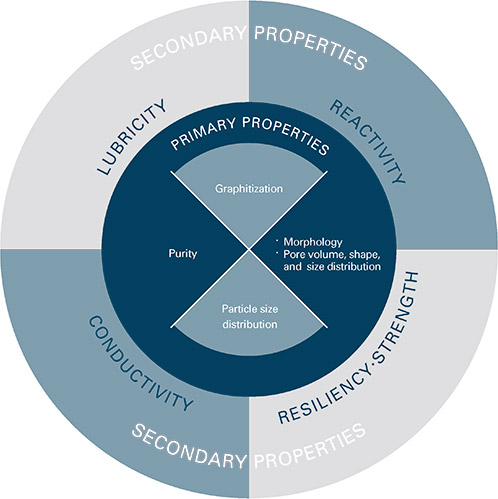
What is graphite? - ECGA

About Graphite - Graphite Products

DIAMOND Vs GRAPHITE Diamond and - Kannan's Chemistry page
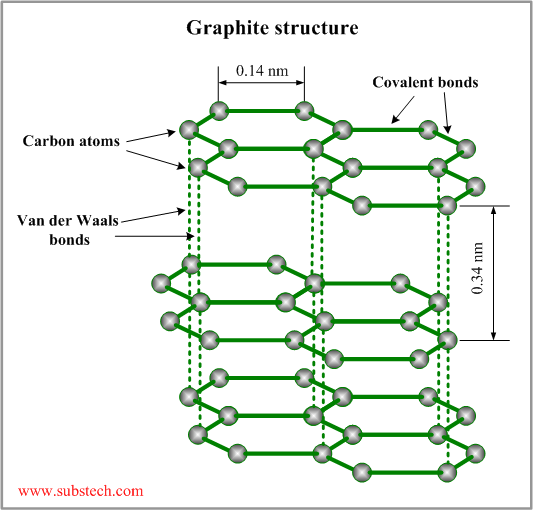
Graphite [SubsTech]
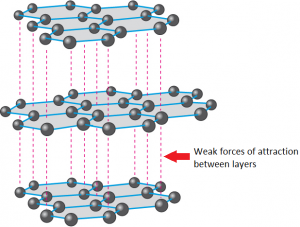
1:50 explain how the structures of diamond, graphite and C60 fullerene influence their physical properties, including electrical conductivity and hardness - TutorMyself Chemistry

UNM Foundation Engineering - Do you know that diamond and graphite are made from the same element? Source: . . . . . . #WeAreUoN #UoNMalaysia #UNM #nottinghammalaysia #nottinghamuniversity

Solved 4. Answer all parts (a) Use hybridization to account
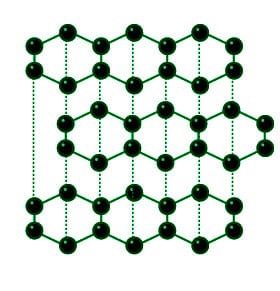
Diamond and Graphite - Structure, Uses, Properties, Applications - GeeksforGeeks
giant covalent structures

/pub/media/catalog/product/2/b/2bc76e2e_7503_4557_bc52_4e9435f83fda_3b4e.jpg)







