IQ OQ PQ, Process Validation, Equipment Validation
IQ OQ PQ are 3 pillars of Process Validation. IQ stands for Installation Qualification. OQ is Operational Qualification and PQ is Performance Qualification.
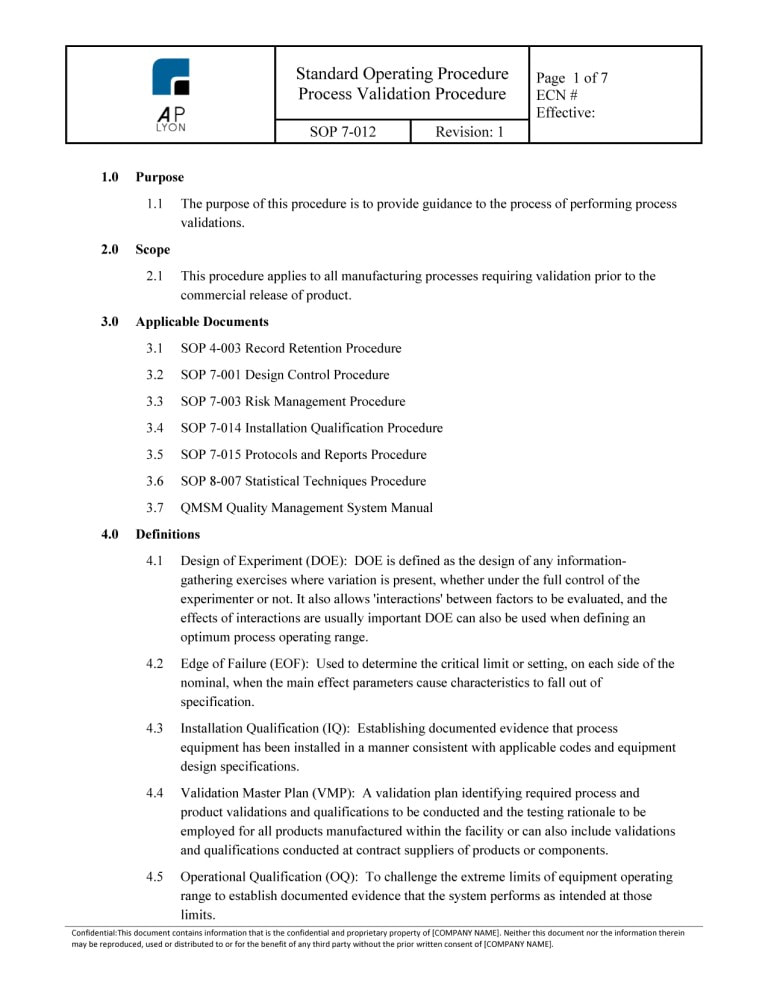
ISO 13485 Process Validation Procedure Bundle
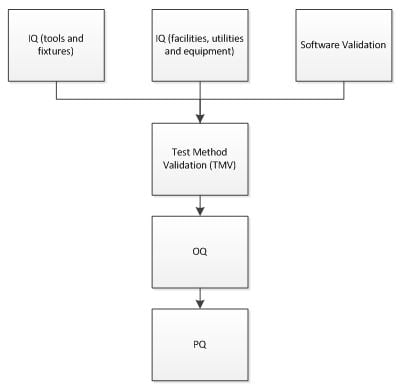
Operational Qualifications 'Worst Case' Conditions

Equipment Qualification - IQ, OQ, PQ Protocols : Compliance Training Webinar (Online Seminar)
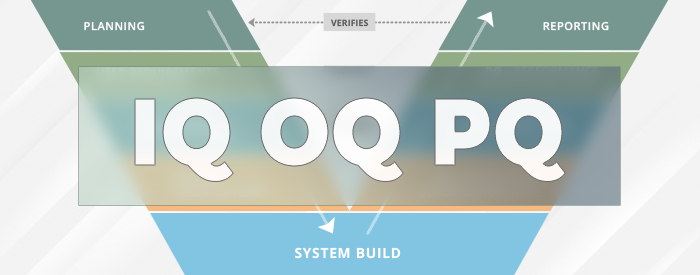
What is IQ OQ PQ in Software Validation?
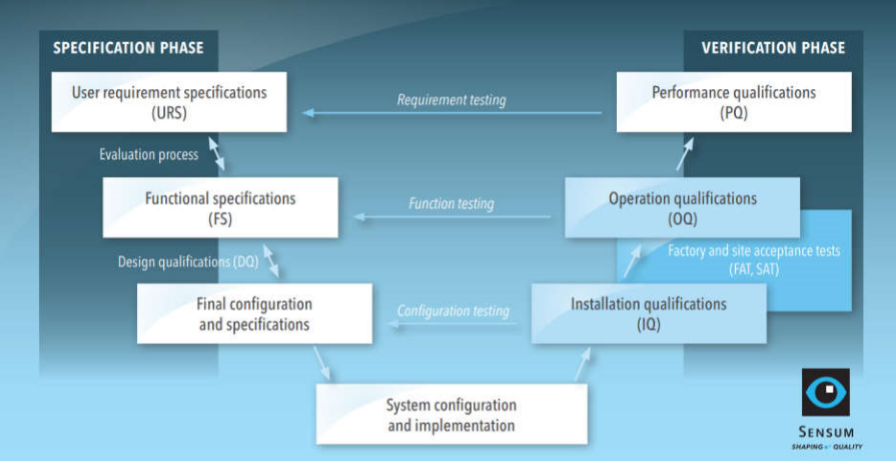
Pharmaceutical qualification and validation: tips to get through nightmares

Validation and Qualification in Laboratory Analytics - Krüss laboratory equipment

IQ, OQ, PQ: what's needed for equipment validation in life sciences?
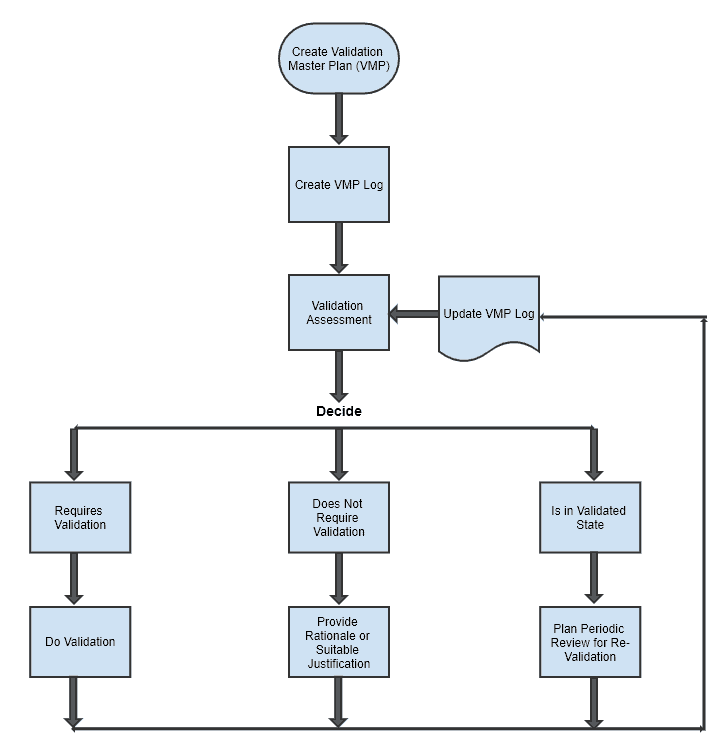
Process Validation: The Essential Guide to Ensuring Product Quality and Compliance - Pharma GxP

Six Sigma Validation Process - Taylor Enterprises

Mastering Process Validation: An In-Depth Guide to IQ/OQ/PQ in Biomanufacturing

Validation Qualifications IQ OQ PQ : PresentationEZE

Do you know Process Validation?

What is a master validation plan