Structured Benefit-Risk Assessment of a New Quadrivalent Meningococcal Conjugate Vaccine (MenACYW-TT) in Individuals Ages 12 Months and Older
Structured Benefit-Risk Assessment of a New Quadrivalent Meningococcal Conjugate Vaccine (MenACYW-TT) in Individuals Ages 12 Months and Older

Immunogenicity and safety of meningococcal group A, C, W and Y tetanus toxoid conjugate vaccine: review of clinical and real-world evidence
PDF) Structured Benefit-Risk Assessment of a New Quadrivalent Meningococcal Conjugate Vaccine (MenACYW-TT) in Individuals Ages 12 Months and Older

Meningococcal Vaccine: Most Up-to-Date Encyclopedia, News & Reviews
Full article: The changing epidemiology of meningococcal disease in North America 1945–2010

Immunogenicity and safety of meningococcal group A, C, W and Y tetanus toxoid conjugate vaccine: review of clinical and real-world evidence
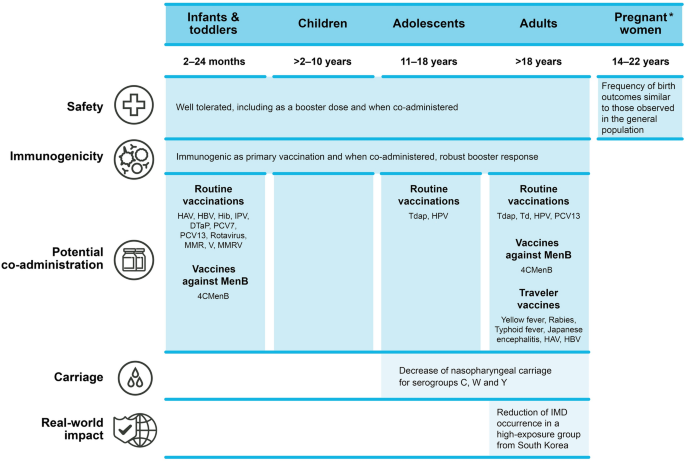
A Decade of Fighting Invasive Meningococcal Disease: A Narrative Review of Clinical and Real-World Experience with the MenACWY-CRM Conjugate Vaccine
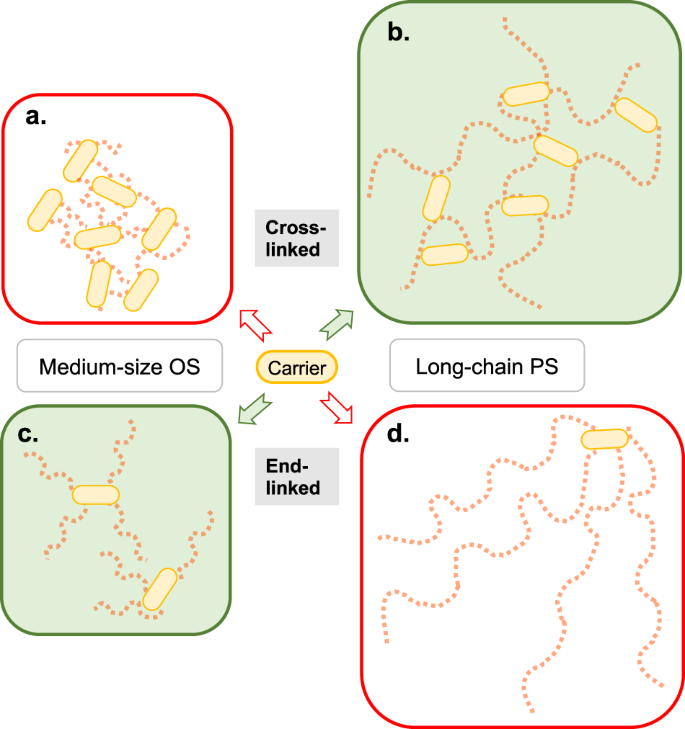
Combined effects of glycan chain length and linkage type on the immunogenicity of glycoconjugate vaccines
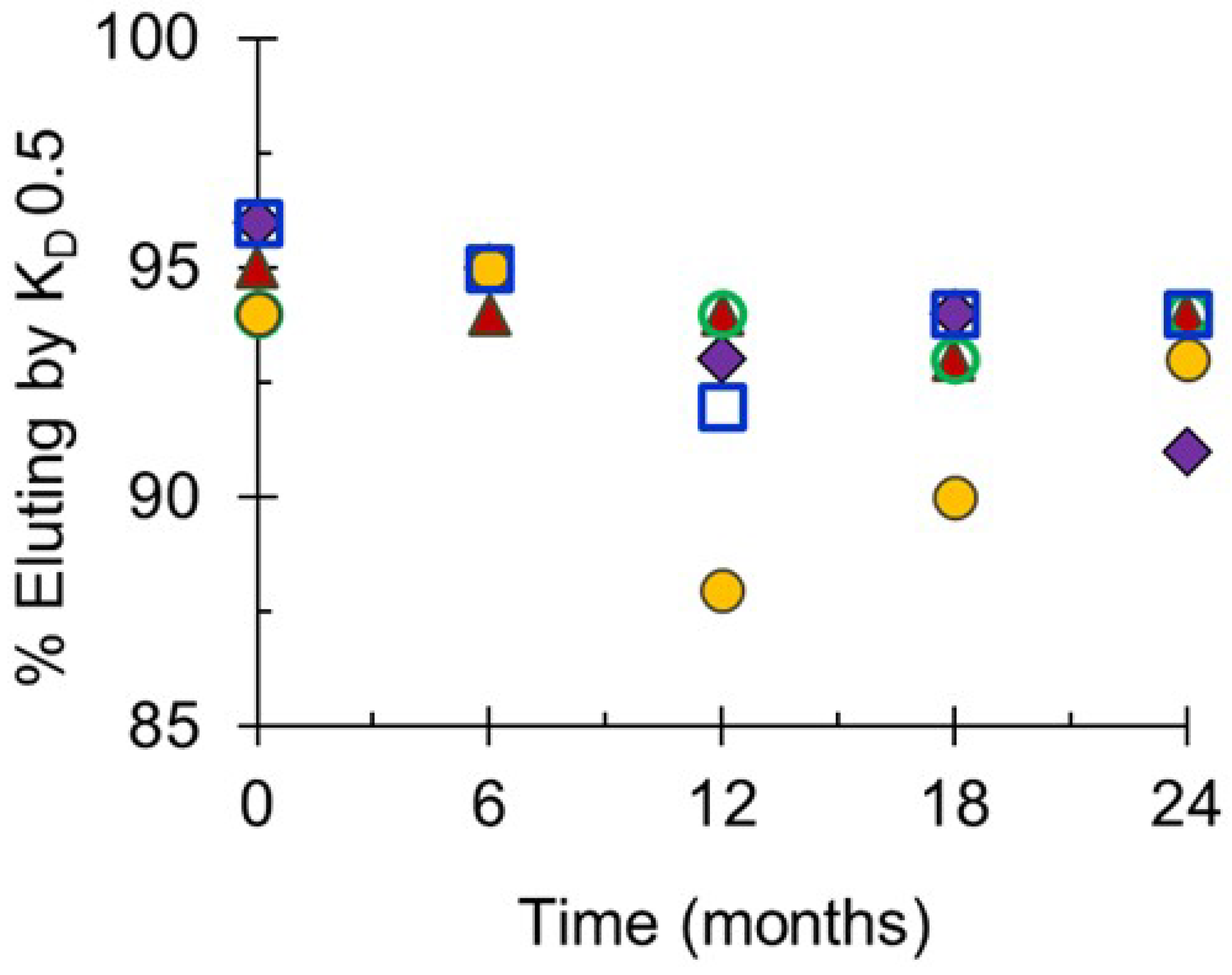
Pathogens, Free Full-Text

Structured Benefit-Risk Assessment of a New Quadrivalent Meningococcal Conjugate Vaccine (MenACYW-TT) in Individuals Ages 12 Months and Older
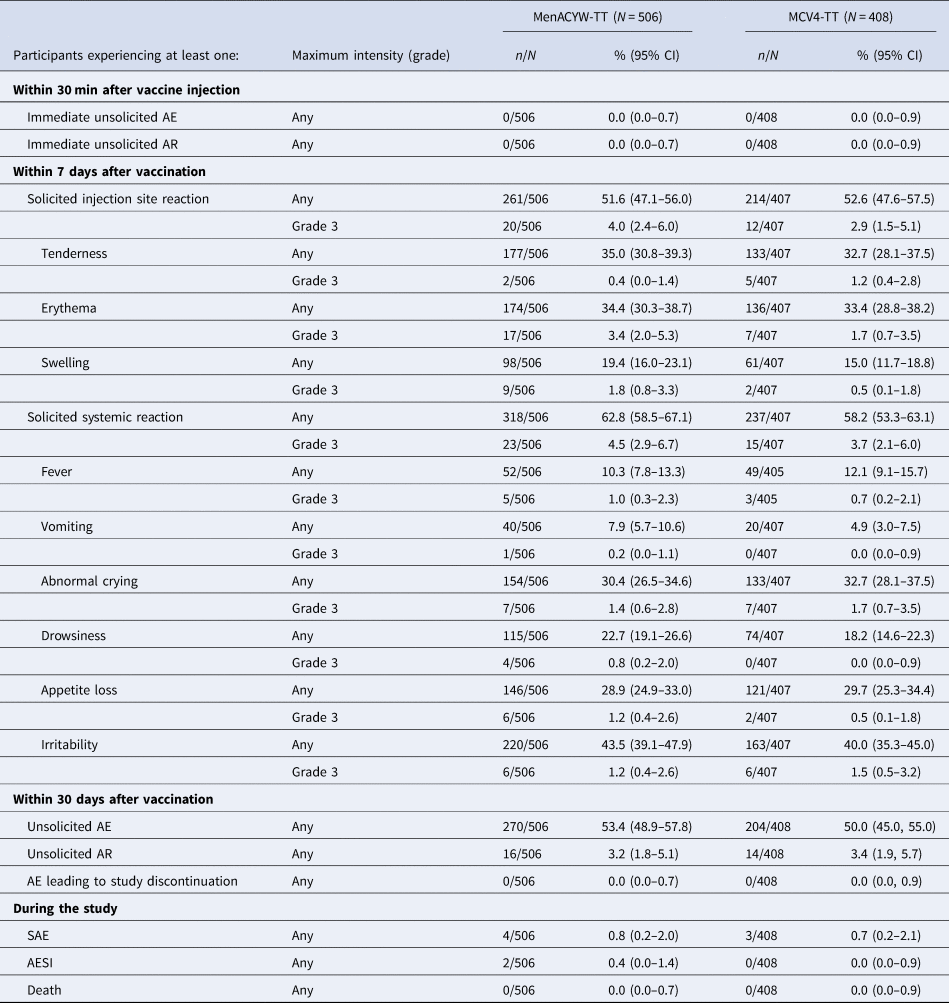
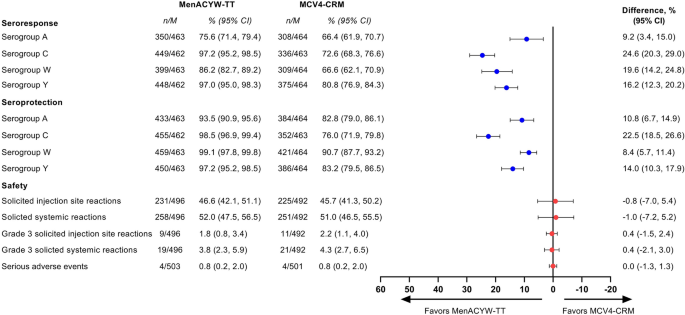
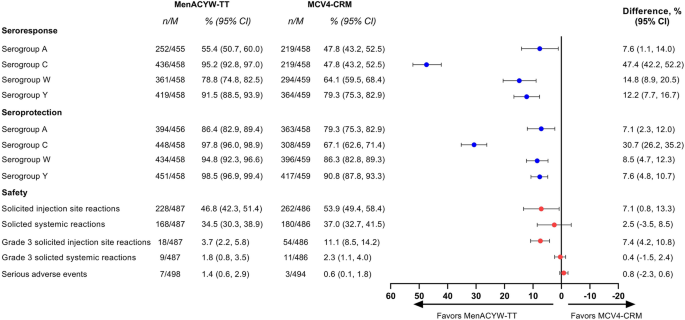
Immunogenicity and safety of a quadrivalent meningococcal tetanus toxoid-conjugate vaccine (MenACYW-TT) vs. a licensed quadrivalent meningococcal tetanus toxoid-conjugate vaccine in meningococcal vaccine-naïve and meningococcal C conjugate vaccine






:watermark(cdn.texastribune.org/media/watermarks/2012.png,-0,30,0)/static.texastribune.org/media/videos/080912_Insider_Art.jpg)