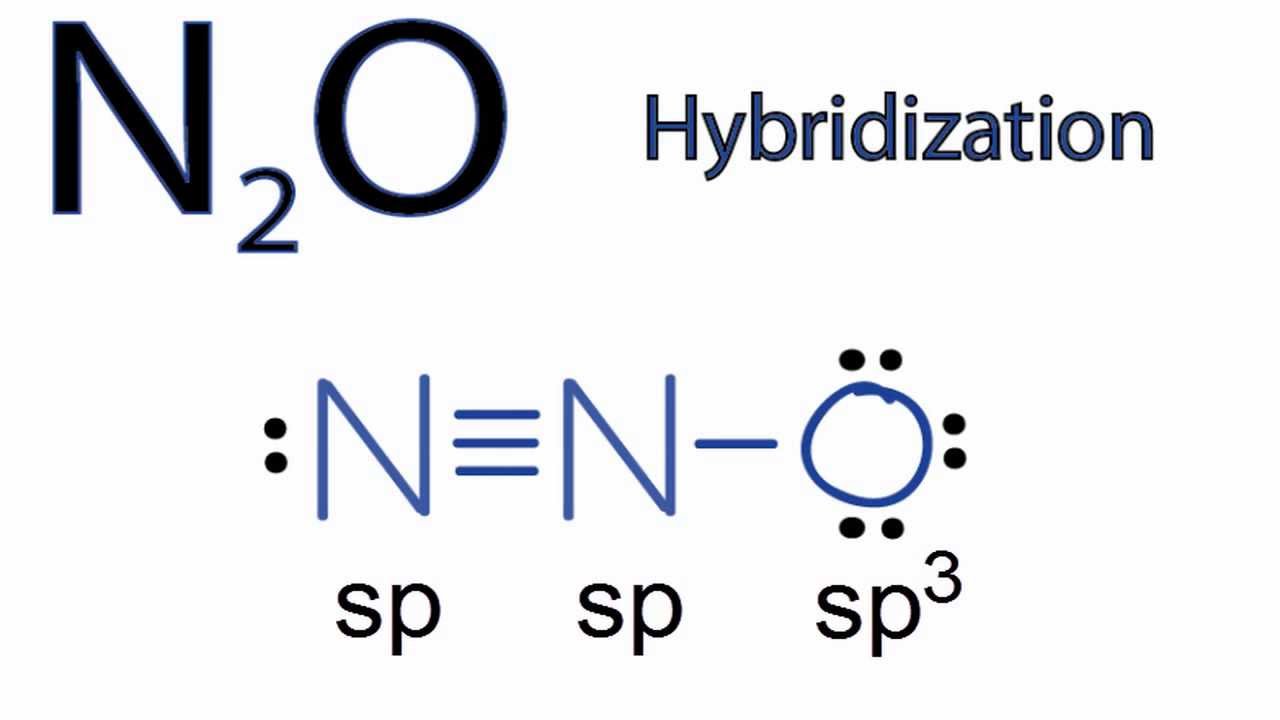
N2O Hybridization: Hybrid Orbitals for N2O

Solved 1. Consider the following Lewis structures for

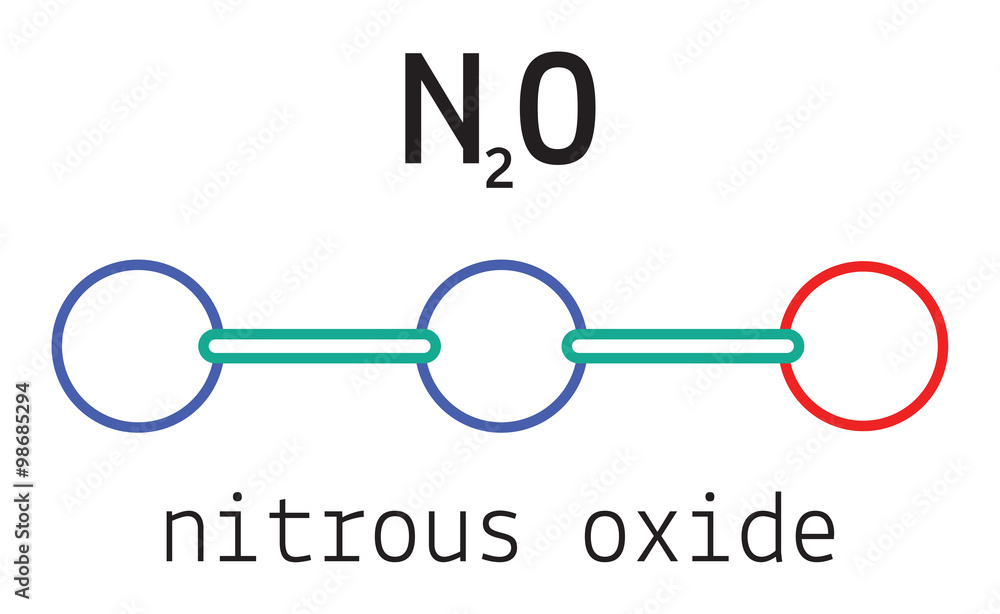
N2O Hybridization: Hybrid Orbitals for N2O

Solved 10. What is the formal charge on the nitrogen atom in
What is the hybridization of N in NO? - Quora

Nitrous oxide, N_2O, has a linear structure NNO. Write resonance formulas for this molecule and from them estimate the NN bond length in the molecule.

N2O Lewis Structure, Molecular Geometry, Hybridization, and MO Diagram - Techiescientist

NO2 Hybridization (Nitrogen Dioxide)
What is the hybridisation of central atom in N20 (nitrous oxide).

Chemistry Made Easy: N2O Lewis Structure and Molecular Geometry

Three non-equivalent resonance structures are possible for nitrous oxide ( N2O), laughing gas. a. Draw the three resonance structures and indicate the formal charge on each atom in each resonance structure. b. Based

c) Would you expect N2O to exhibit delocalized p bonding?
Why is N the central atom in N2O? - Quora

Spectroscopy and Infrared Photofragmentation Dynamics of Mixed Ligand Ion–Molecule Complexes: Au(CO)x(N2O)y+