Quadrivalent Meningitis (MCV4)

Phase 3 Clinical Trial results demonstrate promising efficacy of Meningitis vaccine

Meningitis Vaccine - e7 Health
FDA OKs Menactra as Booster Against Meningococcal Disease

Search strategy and flow diagram summarizing study selection.
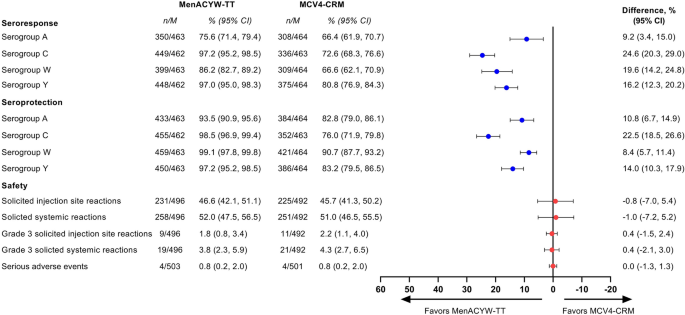
Structured Benefit-Risk Assessment of a New Quadrivalent Meningococcal Conjugate Vaccine (MenACYW-TT) in Individuals Ages 12 Months and Older
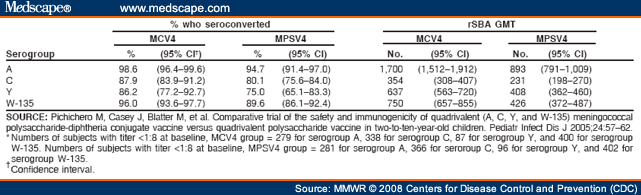
Not Recommend Routine Vaccination of All Children Aged 2-10 Years With MCV4
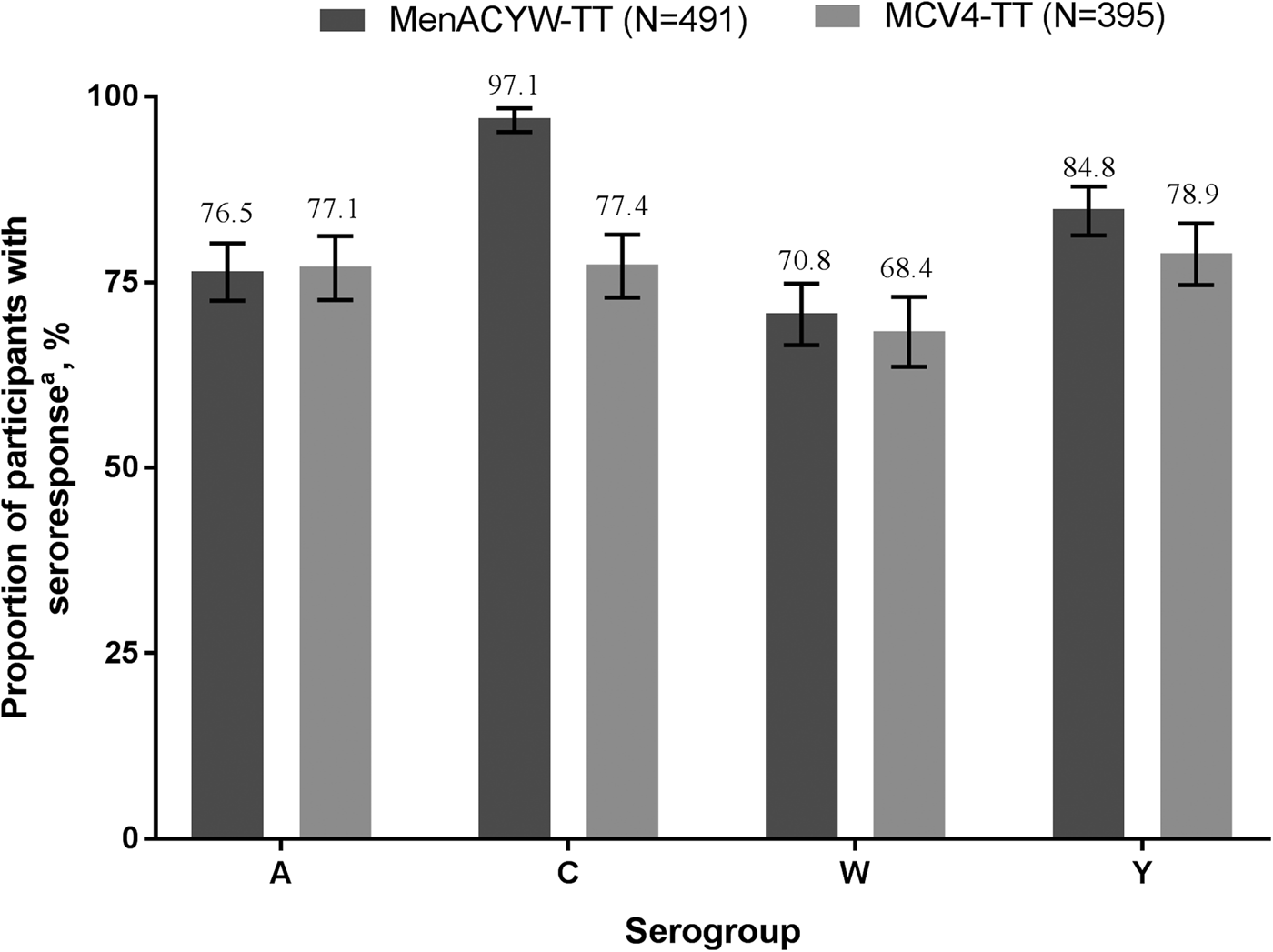
Immunogenicity and safety of a quadrivalent meningococcal tetanus toxoid-conjugate vaccine (MenACYW-TT) vs. a licensed quadrivalent meningococcal tetanus toxoid-conjugate vaccine in meningococcal vaccine-naïve and meningococcal C conjugate vaccine
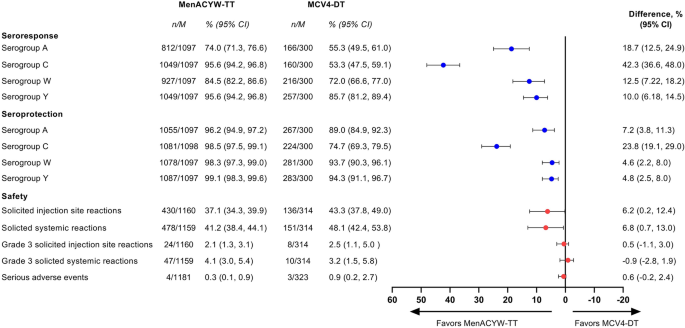
Structured Benefit-Risk Assessment of a New Quadrivalent Meningococcal Conjugate Vaccine (MenACYW-TT) in Individuals Ages 12 Months and Older

Global Meningococcal Vaccine Market- Growth, Trends, Covid-19 Impact & Forecast
The meningococcal conjugate vaccine: Uses, Side Effects, Dosage & Reviews

Haemophilus Influenzae Type B Meningococcus Tetanus Vaccine - an overview

PDF) Post-Marketing Surveillance Observational Study of Quadrivalent Meningococcal Diphtheria Toxoid Conjugate Vaccine (MenACWY-DT, MCV4/Menactra®) in the Republic of Korea, 2014–2019

Full article: Comparing the meningococcal serogroup C immune response elicited by a tetanus toxoid conjugate quadrivalent meningococcal vaccine (MenACYW-TT) versus a quadrivalent or monovalent C tetanus toxoid conjugate meningococcal vaccine in healthy

Haemophilus Influenzae Type B Meningococcus Tetanus Vaccine - an overview
Meningococcal Vaccine - Infectious Diseases - MSD Manual Professional Edition






.jpg?rev=cf219796ed7e4c12a8d046d97b29be3d)

