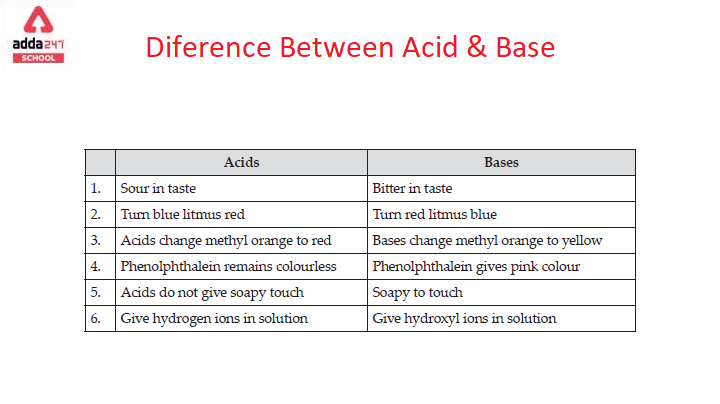
Difference between Strong and Weak Base - with Examples [in Table]
![Difference between Strong and Weak Base - with Examples [in Table]](https://d1avenlh0i1xmr.cloudfront.net/0e323ff3-079c-4d5e-b21f-0f4c25082521/differences-between-strong-and-weak-bases-01.jpg)
Strong BaseWeak BaseThey get completely ionized (split up into ions) in water and produce large amounts of hydroxide ions.These only get partially ionized (split up into ions) in water and produce less amount of hydroxide ions.pH value is close to 14 but smaller than it.pH value is closer to 7 but
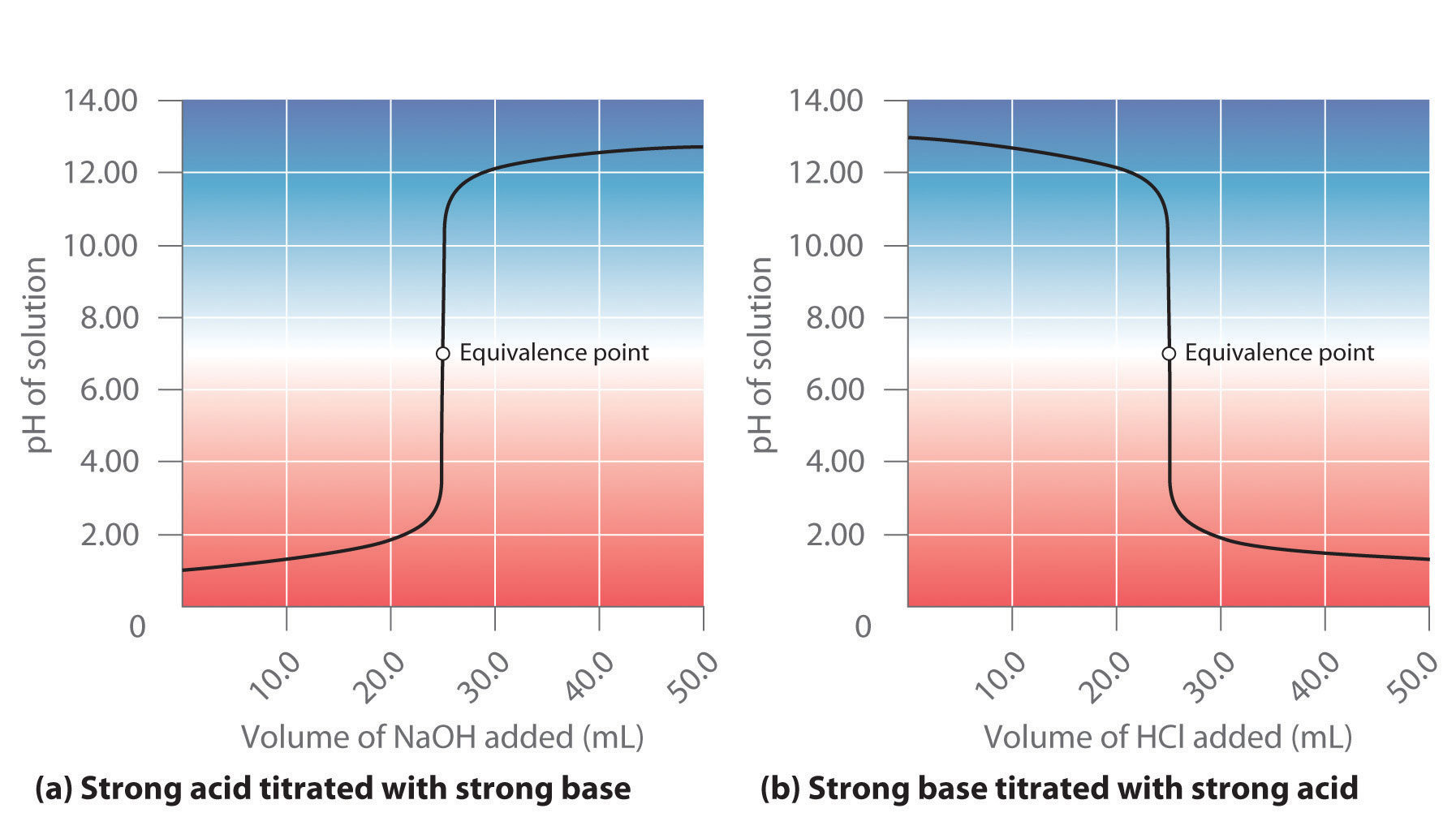
Acid–Base Titrations
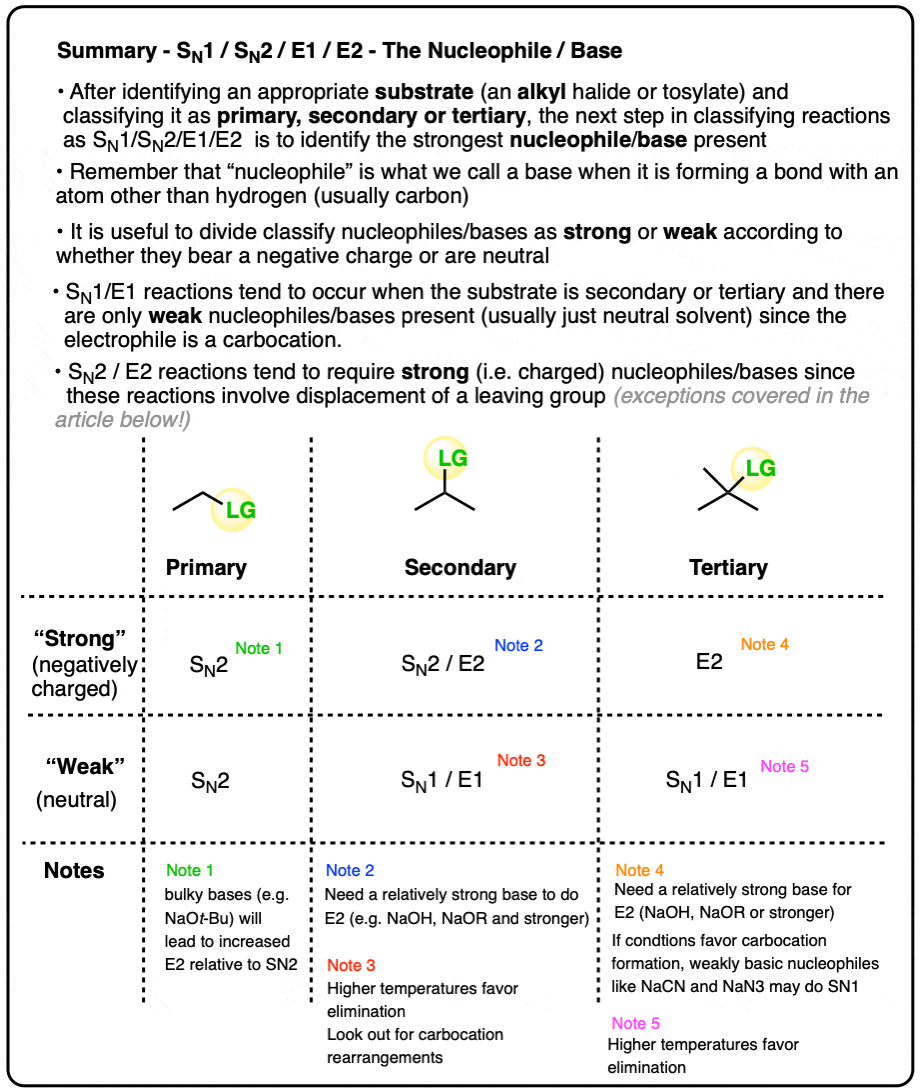
Deciding SN1/SN2/E1/E2 (2) - The Nucleophile/Base
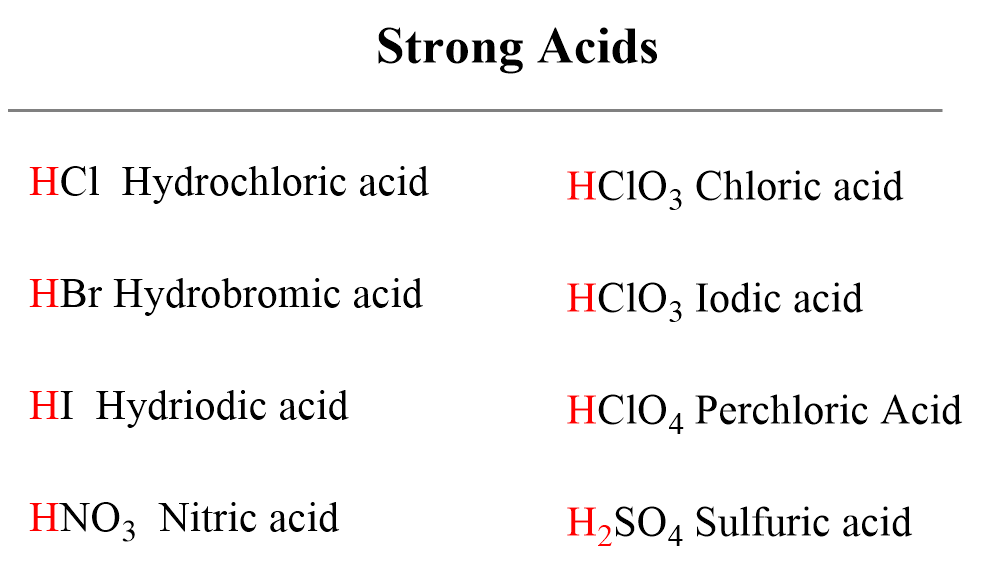
Acid Strength, Ka, and pKa - Chemistry Steps
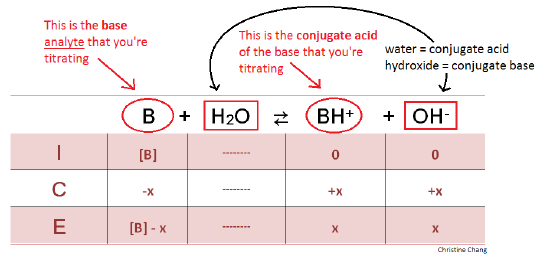
Titration of a Weak Base with a Strong Acid - Chemistry LibreTexts

2.5: Weak Acids and Weak Bases - Chemistry LibreTexts
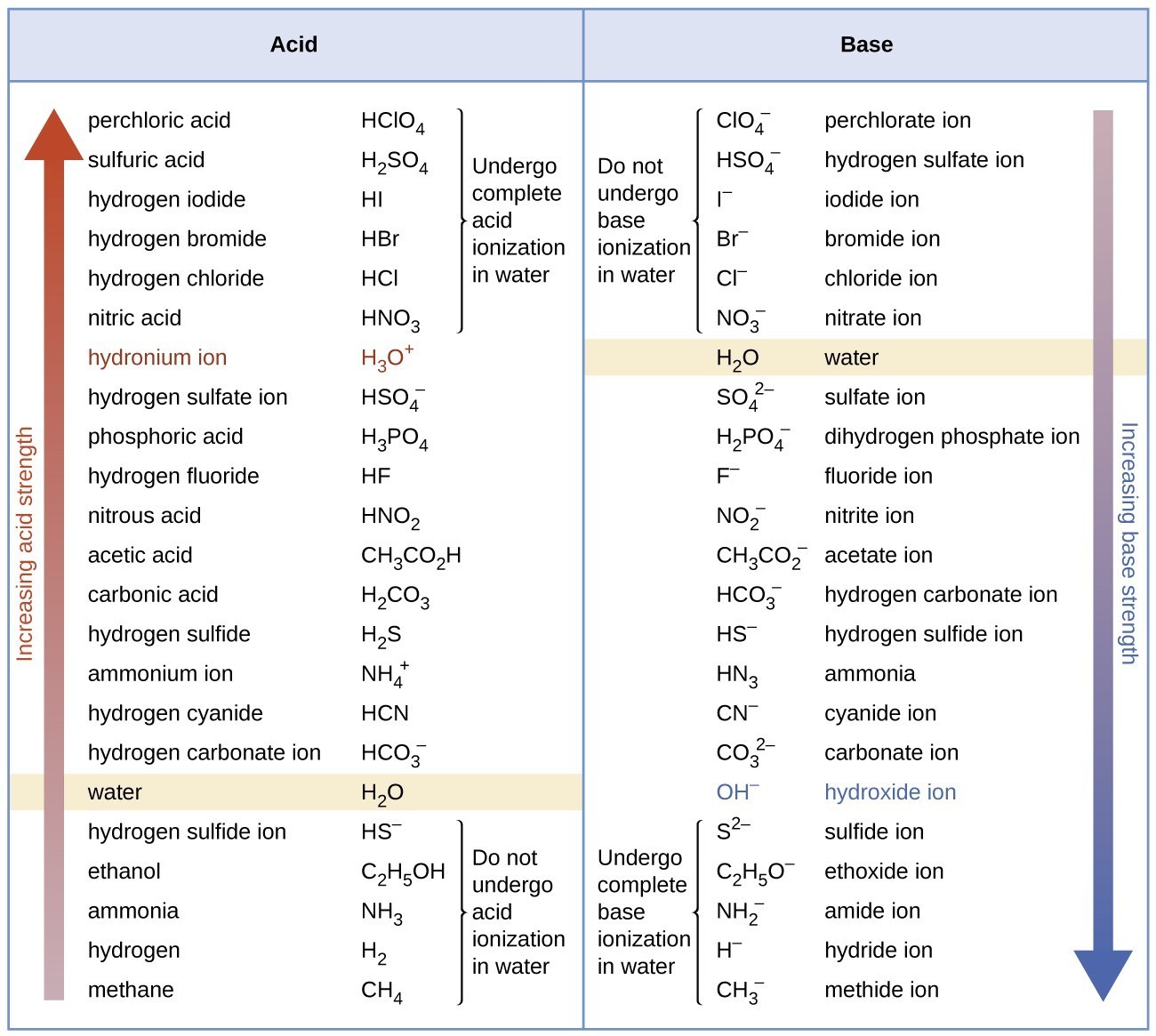
Relative Strengths of Acids and Bases
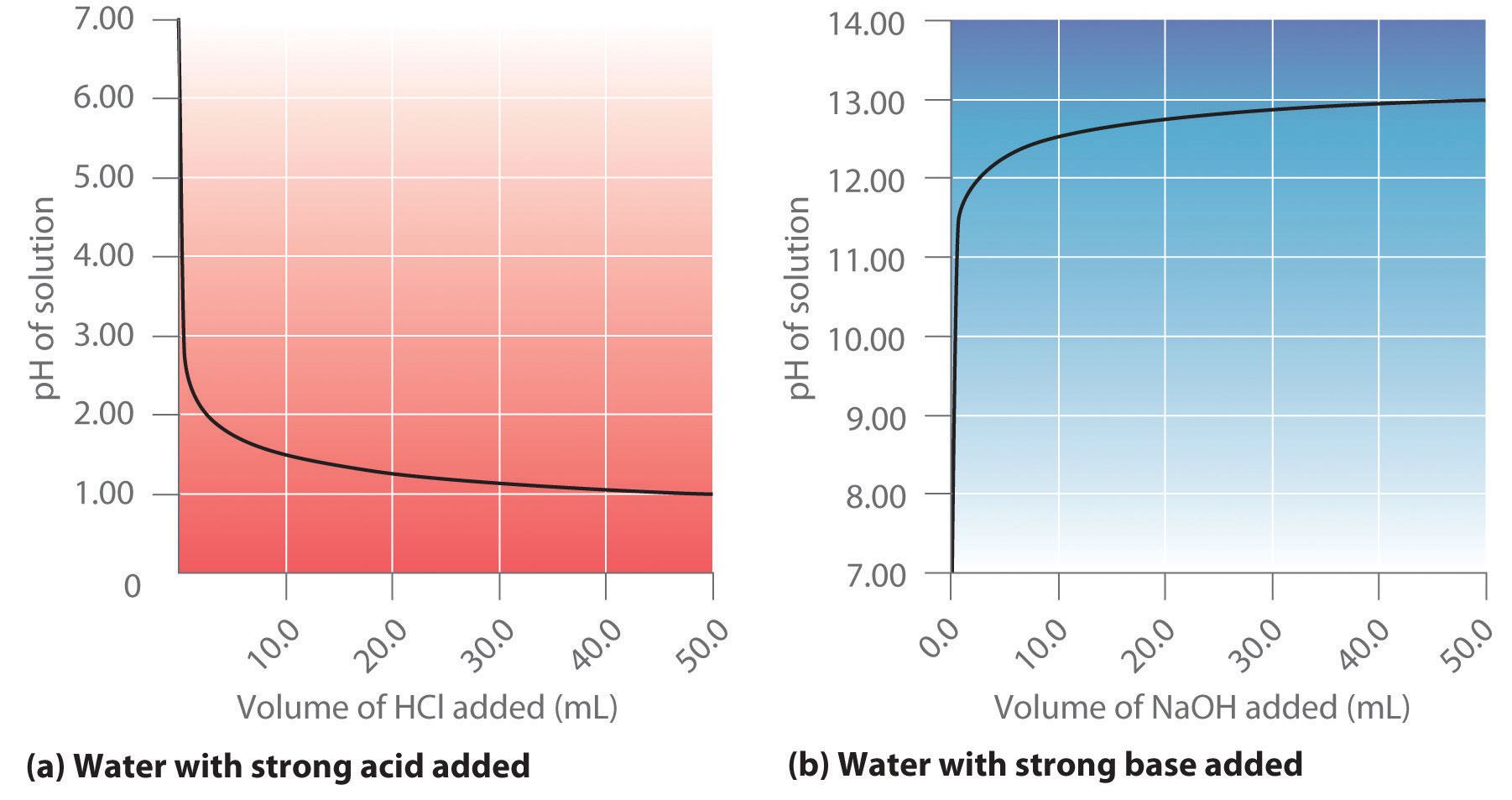
Acid–Base Titrations
.png)
What are Bases? - Definition, Examples, Types, Properties and Uses - GeeksforGeeks

Strong Acids & Bases, Table, Formula & Examples - Video & Lesson Transcript
31. What is the pH of salt of strong acid and weak base?

Titration curves & equivalence point (article)

Here is an example of a titration curve, produced when a strong base is added to a strong acid. This curve shows how pH varies as 0.100 M NaOH is added to 50.0 mL of 0.100 M HCl.
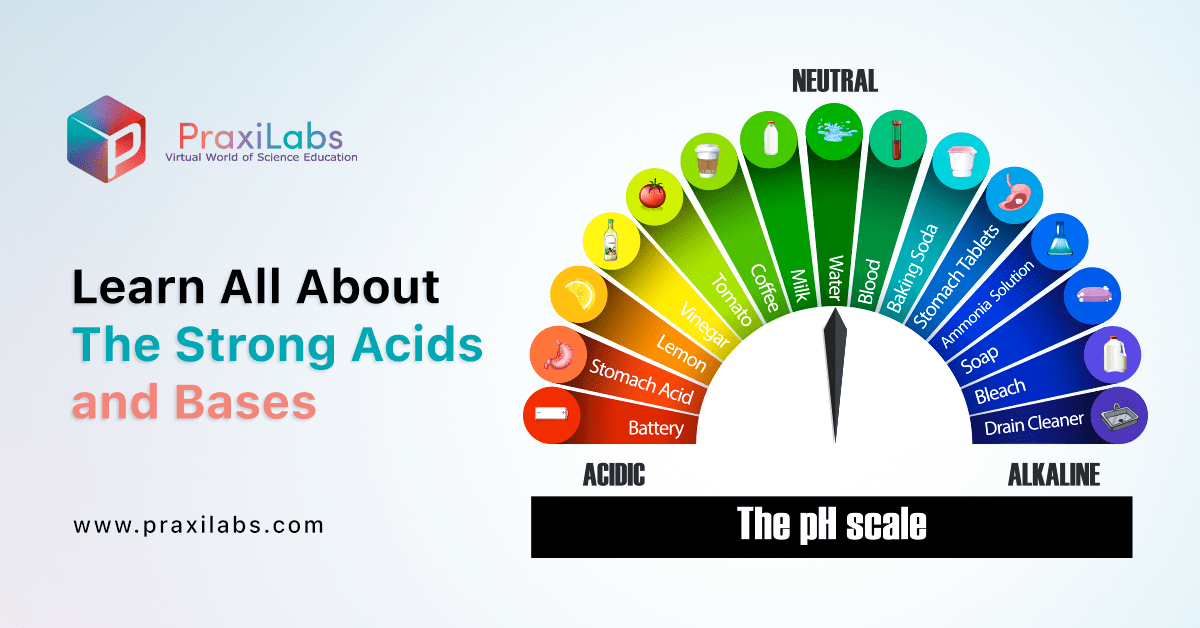
Learn All About The Strong Acids and Bases - PraxiLabs




:strip_icc()/bissell-little-green-pet-deluxe-portable-carpet-cleaner-tout-2000-b5ce2536b9814118886694c4becc8dc5.jpg)