The Submission Dossier Regulatory Affairs in Latin America
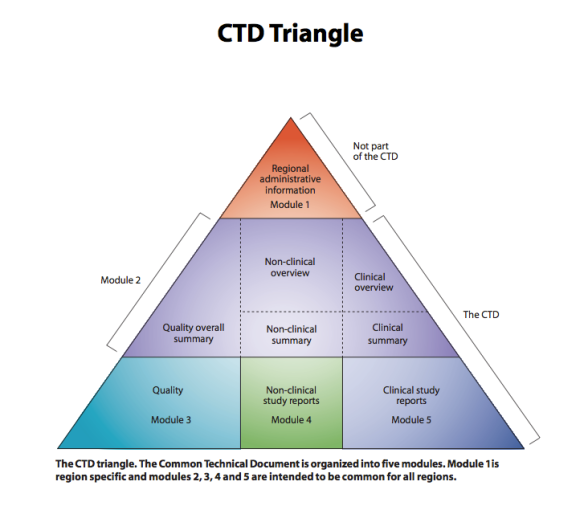
The submission dossier is the packet of documents that are to be submitted to a health authority for registration of a product, or for other life-cycle maintenance activities, such as renewal of registration or CMC variations. The requirements vary very widely from country to country, but in general a dossier contains administrative documents, (such as…

Registration and post-approval variation of pharmaceutical drugs in Latin America: challenges and opportunities, Journal

Manager Regulatory Affairs Resume Samples

Regulatory Affairs

Regulatory Affairs Manager Resume Samples
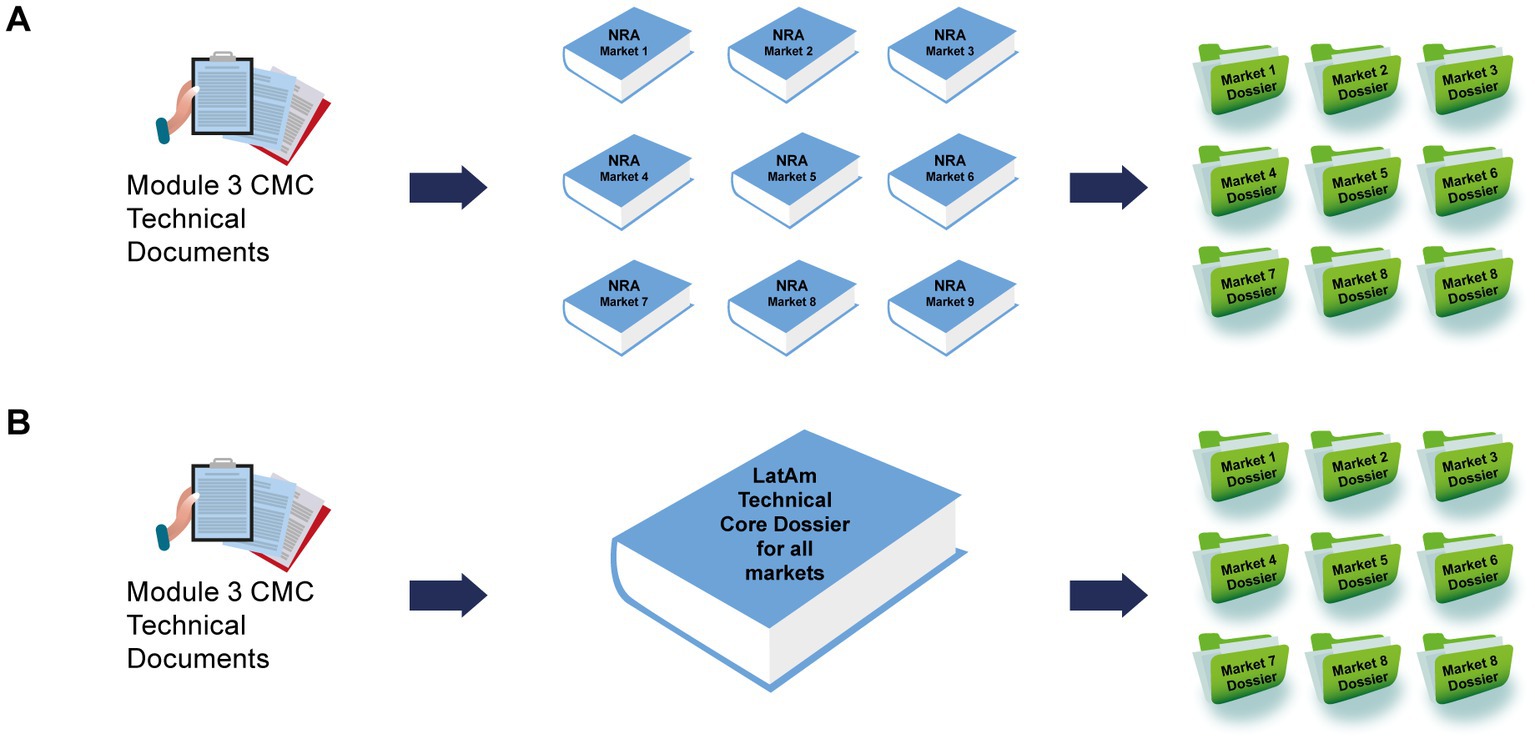
Frontiers Establishing a core dossier for multiple regulatory submissions: a case study in the Latin America region

Regulations: Central and South America - AgriBusiness Global

Regulatory Affairs - Pharmaceutical Technology
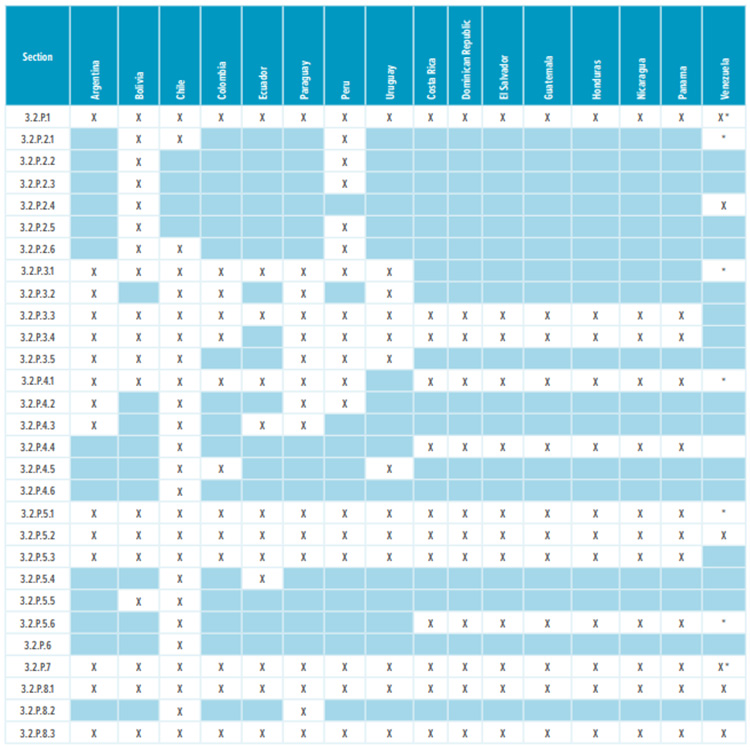
CMC Requirements for New Drug Registration in Latin America

PDF) REGULATORY REQUIREMENTS FOR REGISTRATION OF GENERIC DRUGS IN “BRICS” COUNTRIES

PDF] A comprehensive study on regulatory requirements for development and filing of generic drugs globally









