Understanding The FDA's Current Focus On Risk Evaluation And

lt;p>The FDA recently asked for comments about how the government handles vendor change requests from drug sponsors with risk evaluation and mitigation strategies. So, we asked a REMS expert to help us understand why the agency is focusing on the broad-reaching program and what it could mean for drug manufacturers with REMS products in their portfolios.</p>

2020 at FDA: A Year of Unparalleled Contributions to Public Health

1 Introduction Characterizing and Communicating Uncertainty in
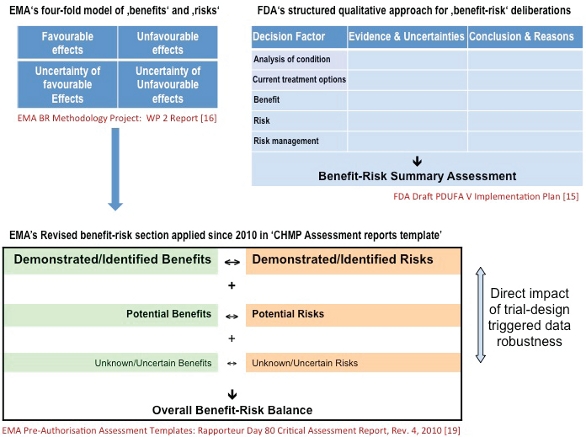
Clinical Quality Risk Management: Growing Impact for Favorable

Reassessing Benefit-Risk: FDA Preps for New Guidance

Necessity of strengthening the current clinical regulatory for

What Is the FDA's Role in Public Health?

FDA releases updated guidelines for risk-based approaches to
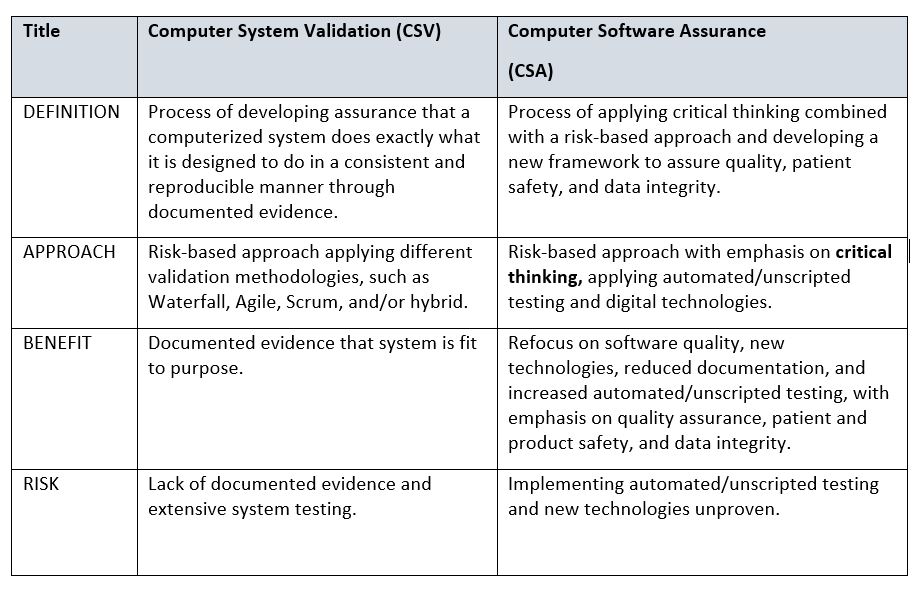
Are You Ready FDA's Transition From Computer System Validation To

The Future of Population‐Based Postmarket Drug Risk Assessment: A
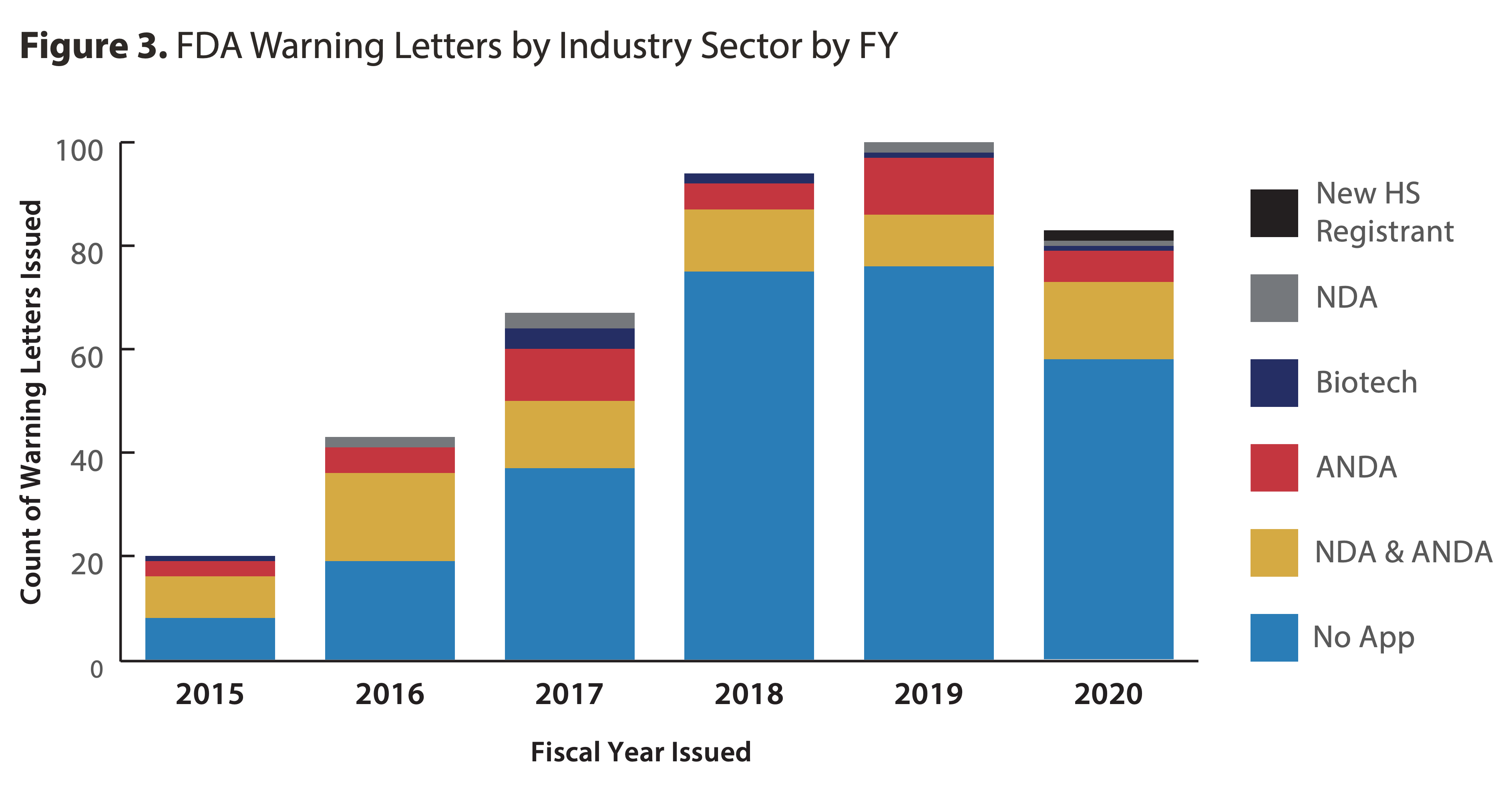
FDA Warning Letter & Inspection Observation Trends [Updated 2023]
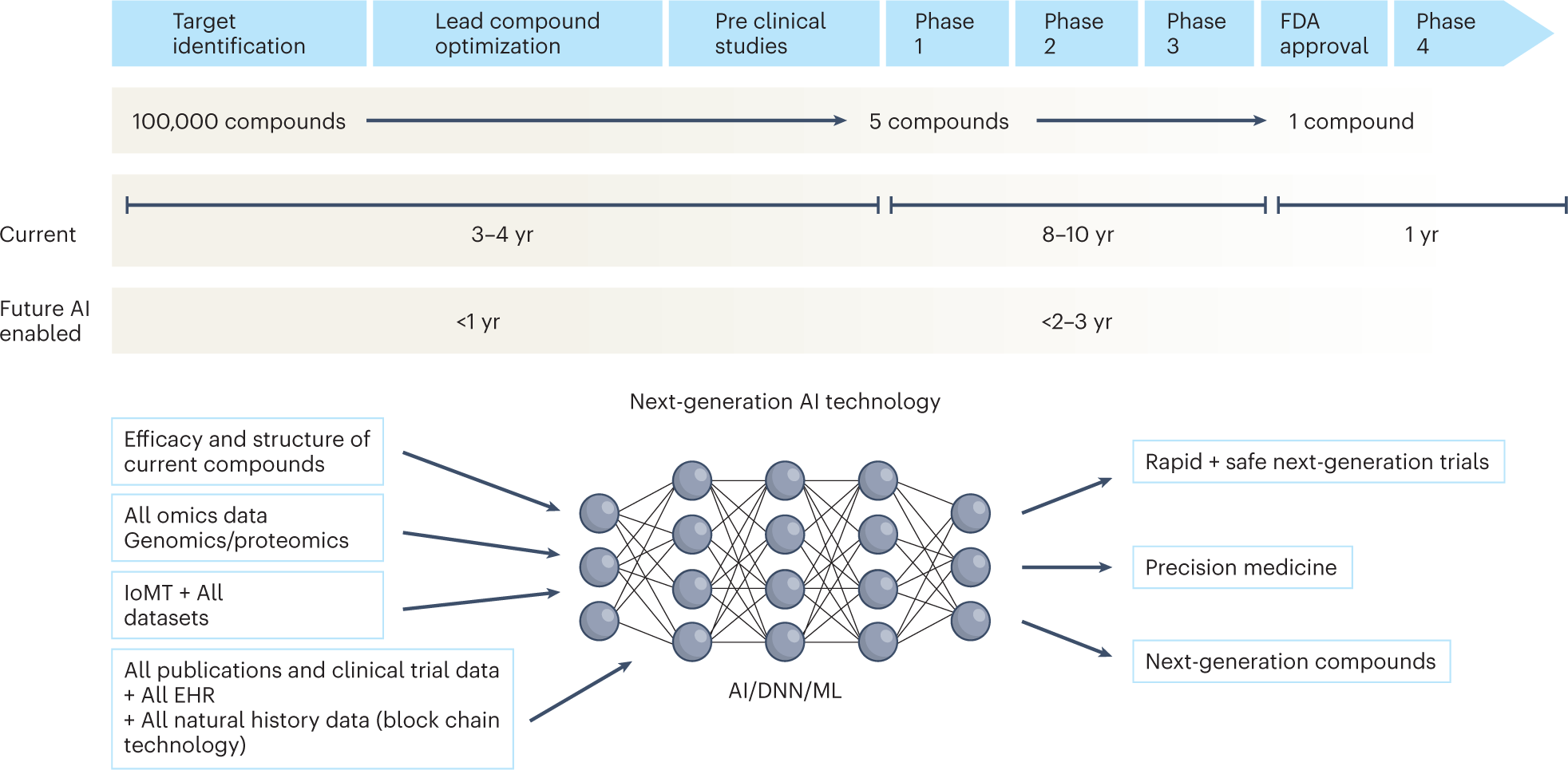
The next generation of evidence-based medicine

2022 drug approvals: Biopharma delivered 34 new drugs

Regulatory Review of Novel Therapeutics — Comparison of Three









