Update on REMS-Required Testing During COVID-19 Pandemic - MPR

“The completion of some REMS-required laboratory testing or imaging studies may be difficult because patients suspected of having COVID-19 may be self-isolating and/or subject to quarantine,” said FDA Principal Deputy Commissioner Amy Abernethy, MD, PhD.

The FDA's Convoluted Stance on Abortion Pills Doesn't Protect Patients — It Endangers Them

Safety and Efficacy of a Third Dose of BNT162b2 Covid-19 Vaccine
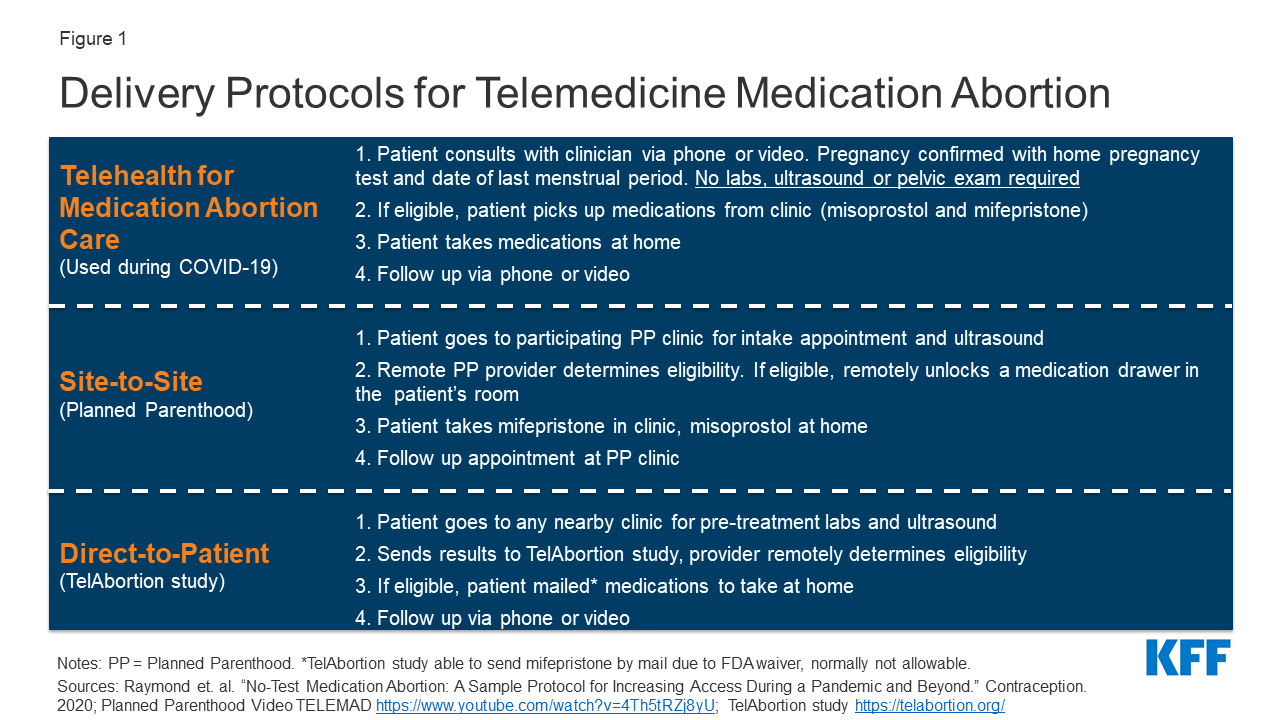
Medication Abortion and Telemedicine: Innovations and Barriers During the COVID-19 Emergency

CA Covid-19 School Readiness Hub
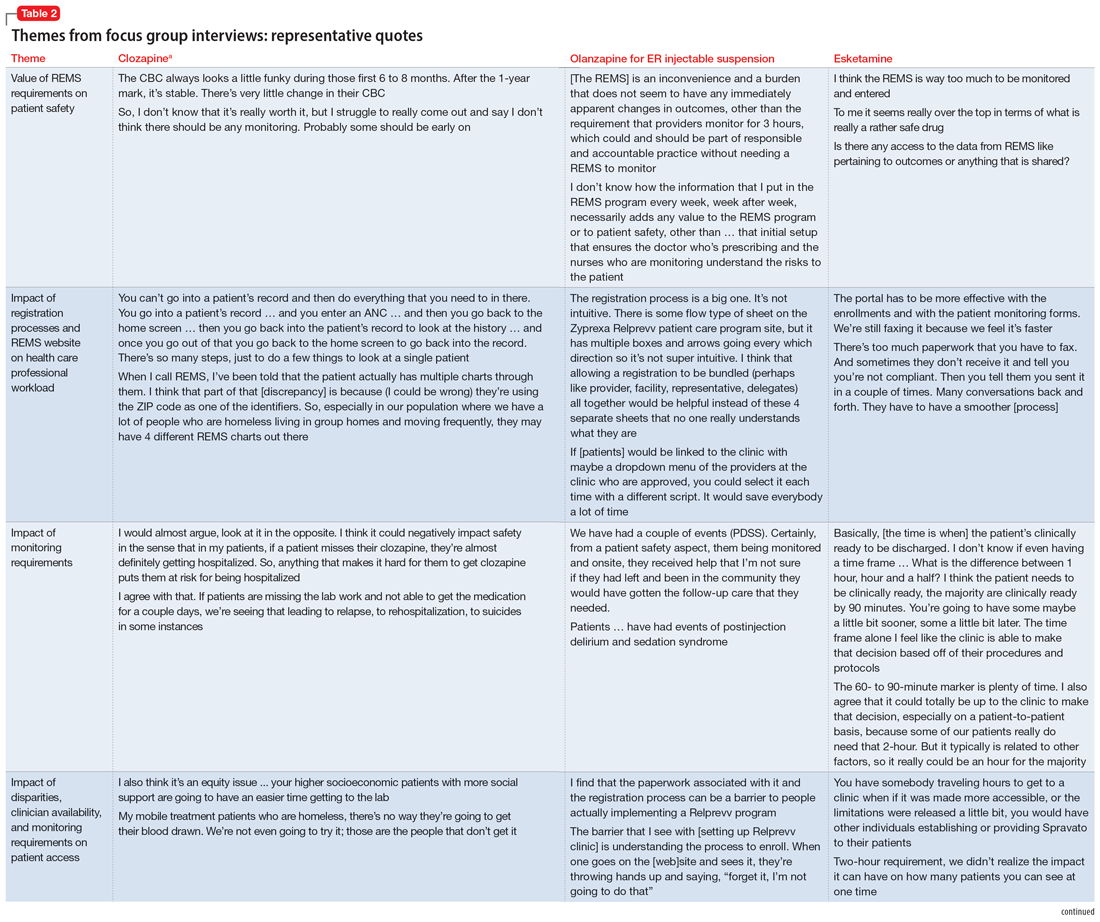
Clozapine During COVID-19: How Best to Ensure Patient Safety

REMS Program & Liver Function JYNARQUE® (tolvaptan) tablets
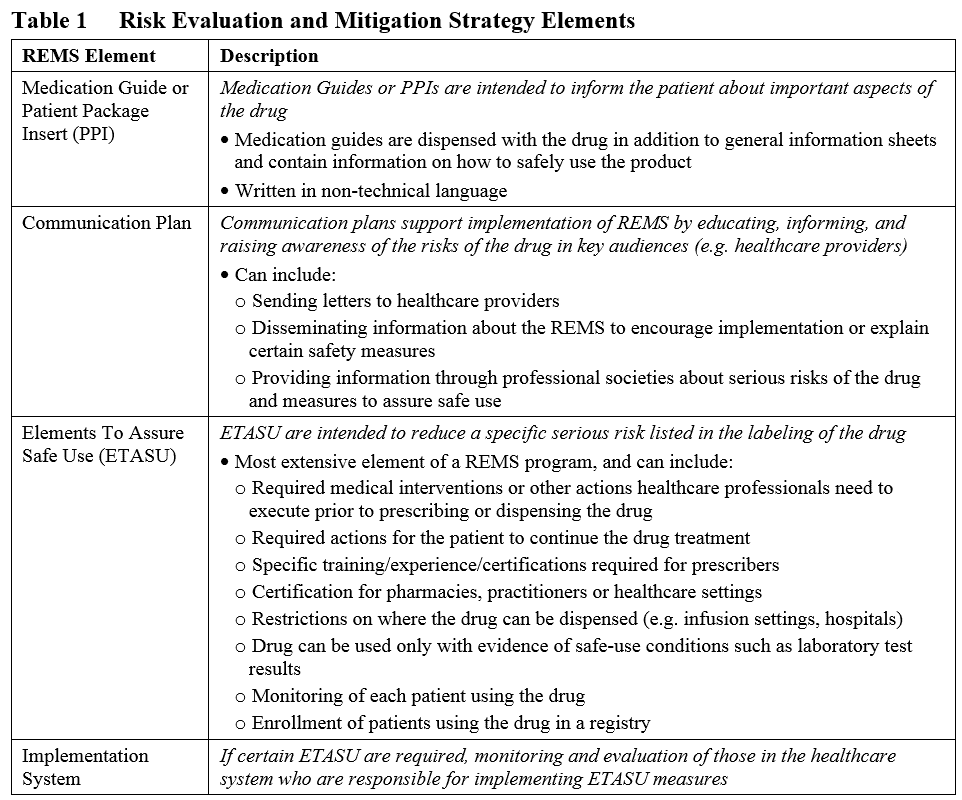
Risk Evaluation and Mitigation Strategy programs: How they can be improved

Lift Unnecessary Restrictions: Access to Medication Abortion During the COVID-19 Pandemic

The utility of the Rapid Emergency Medicine Score (REMS) compared with three other early warning scores in predicting in-hospital mortality among COVID-19 patients in the emergency department: a multicenter validation study

FDA Relaxes Certain Lab and Imaging Test Requirements for REMS Programs During the COVID-19 Pandemic

COVID-19: Federal Efforts Could Be Strengthened by Timely and Concerted Actions

FDA Emergency Use Authorizations

BNT162b2 Protection against the Omicron Variant in Children and Adolescents