Why Is Water a Polar Molecule?
:max_bytes(150000):strip_icc()/GettyImages-1041588324-5c3cf475c9e77c0001d63bca-5c3f692fc9e77c0001d9a10f.jpg)
Water is a polar molecule because the electrons are unevenly distributed. Since the molecule is polar, water is a polar solvent, also.

Properties of Water Polar molecule Hydrogen bonding Surface tension Cohesion and adhesion Universal solvent. - ppt download
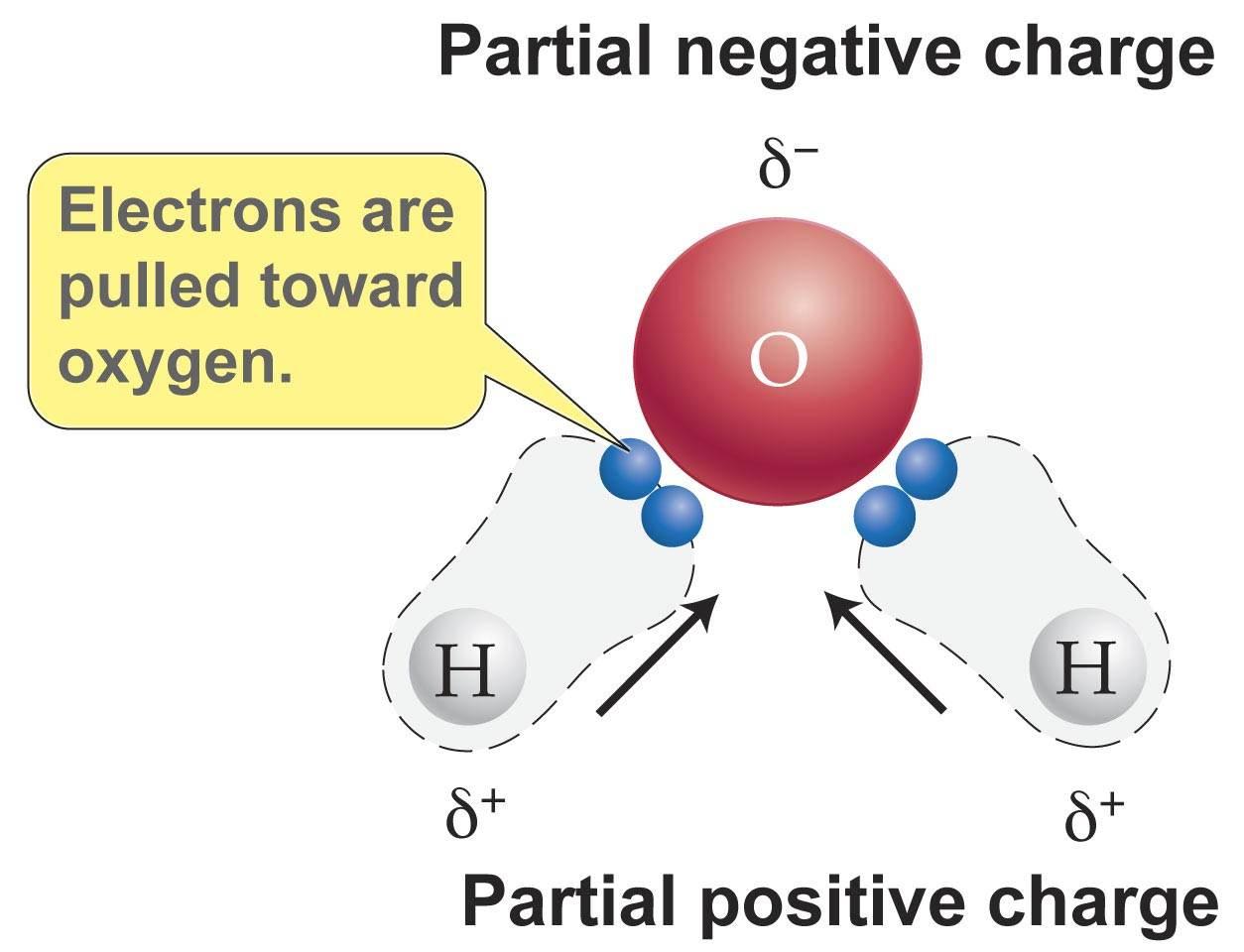
Why is water a polar molecule?

Hydrogen Bond CK-12 Foundation

Lesson Explainer: Polar and Nonpolar Solvents

Properties of Water and pH.pptx - WATER IS A POLAR MOLECULE. WHY? • A WATER MOLECULE IS A POLAR MOLECULE BECAUSE THERE IS AN UNEQUAL SHARING

Chemical Polarity: A Little Bit Of Physics In Your Chemistry - The Institution for Science Advancement
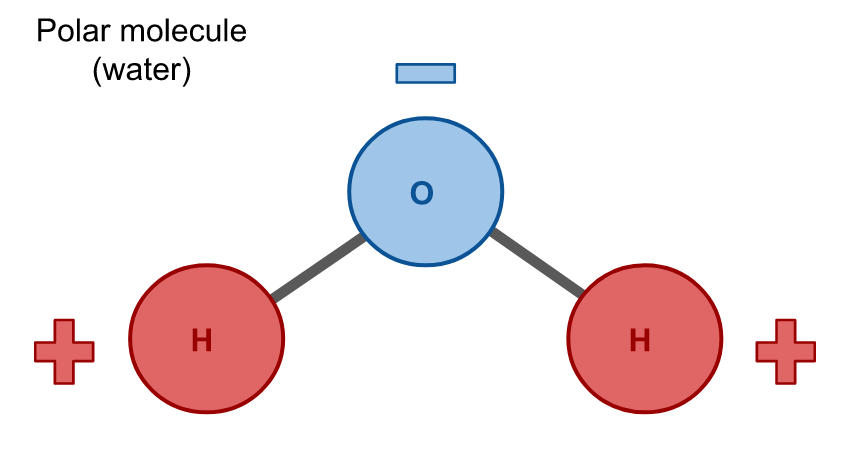
Hydrophobicity
:max_bytes(150000):strip_icc()/some-examples-of-covalent-compounds-603981_final21-a3faebbe543e404fb951d2e789031f56.jpg)
Why Is Water a Polar Molecule?
:max_bytes(150000):strip_icc()/GettyImages-1041588324-5c3cf475c9e77c0001d63bca-5c3f692fc9e77c0001d9a10f.jpg)
Why Is Water a Polar Molecule?