Commissioning, Qualification and Validation for…


Commissioning & Qualification of Equipment and Systems Course
The Difference Between Qualification and Validation - LearnGxP: Accredited Online Life Science Training Courses

Commissioning Qualification Validation CQV

PPT - Commissioning, Qualification and Validation (CQV) - Reasons to Work PowerPoint Presentation - ID:11276506

Installation, Commissioning and Validation
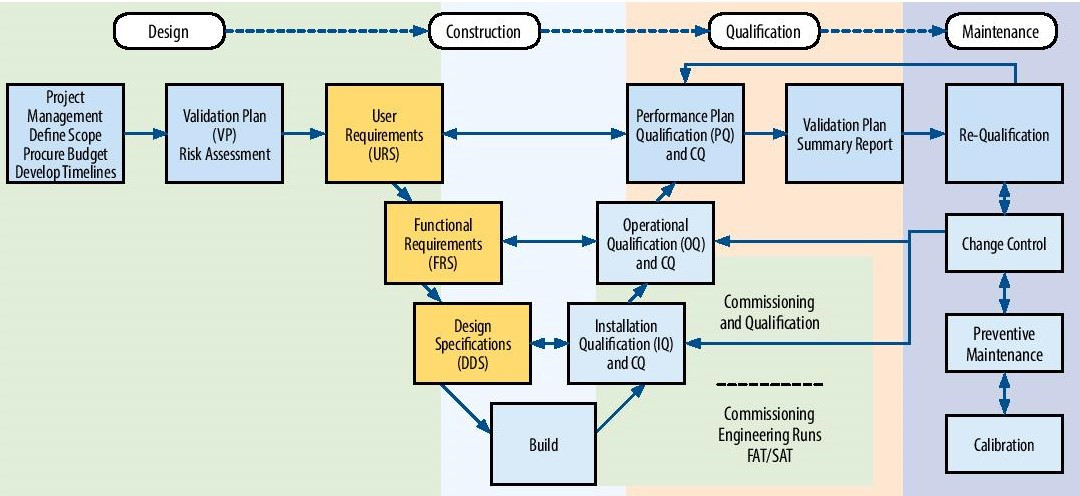
CQV Services - Elomatic India

Pharmaceutical Qualification and Validation Consultants

Guide to commissioning and qualification
Commissioning, Qualification and Validation (CQV) are requirements of modern facilities within the Life Science industry. Be it a Medical Device

Commissioning, Qualification and Validation: A GMP Approach

Commissioning, Qualification and Validation. - CSV Life Science

Will Knapp on LinkedIn: Commissioning, Qualification, and Validation are critical functions in the…

Business Case: Commissioning, Qualification, and Validation (CQV) for Facility, Manufacturing, and Laboratory Equipment and Systems - Kvalito
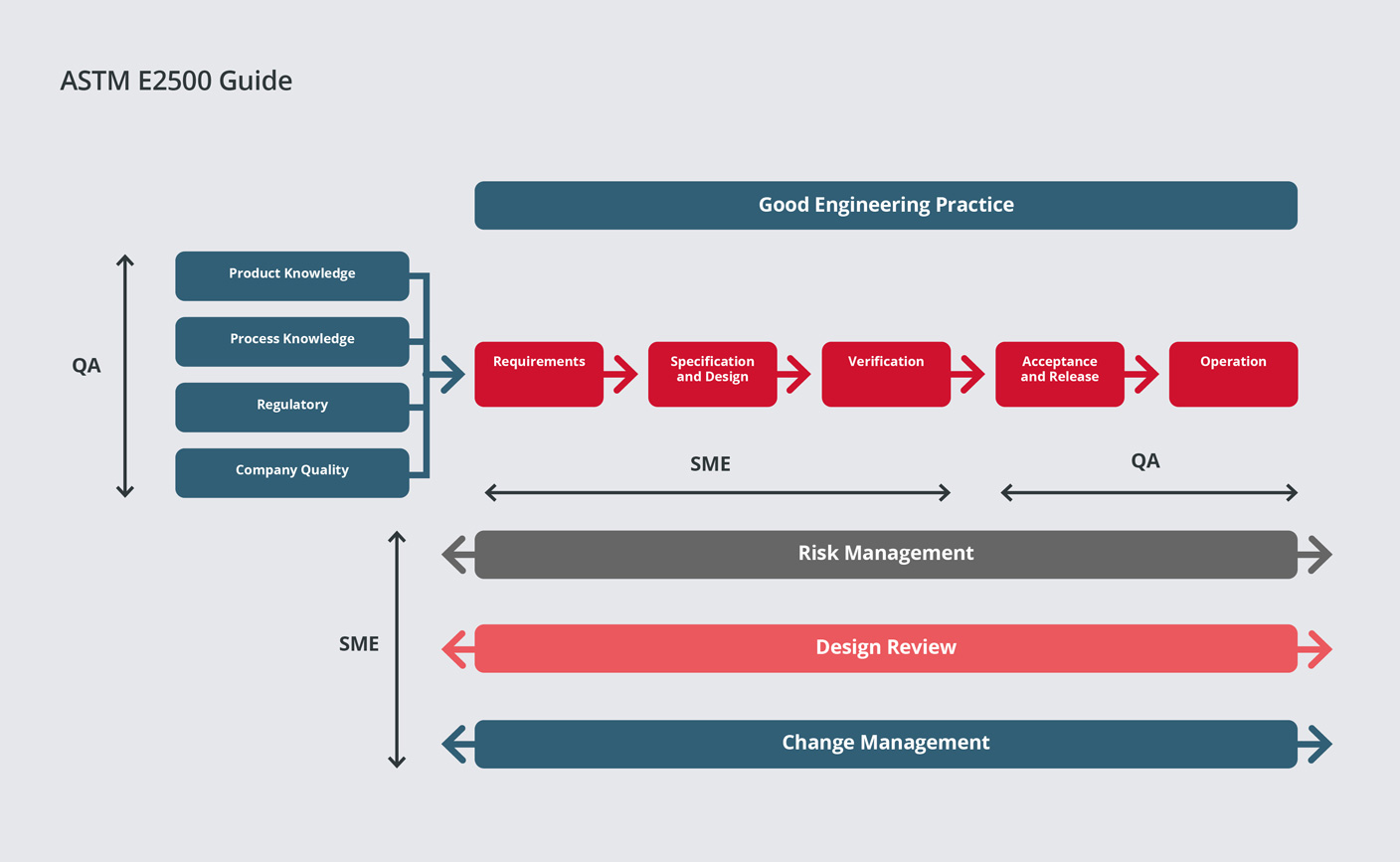
Qualification / Validation - SPGL

Commissioning, Qualification & Validation - IPS









