5.85g of NaCl is dissolved in 1L of pure water. The number of ions

5.85g of NaCl is dissolved in 1L of pure water. The number of ions in 1ml of this solution is

When NaCl is dissolved in water the sodium ion becomes

PDF) Physical Chemistry by P Bahadur abhishek kumar gautam

5.85g of NaCl is dissolved in 1L of pure water. The number of ions in 1ml of this solution is:

5.85g of NaCl is dissolved in 1L of pure water. The number of ions in

5.85 g of NaCl is dissolved in 1 L of pure water . How do you calculate the number of ions in 1mL of this solution?
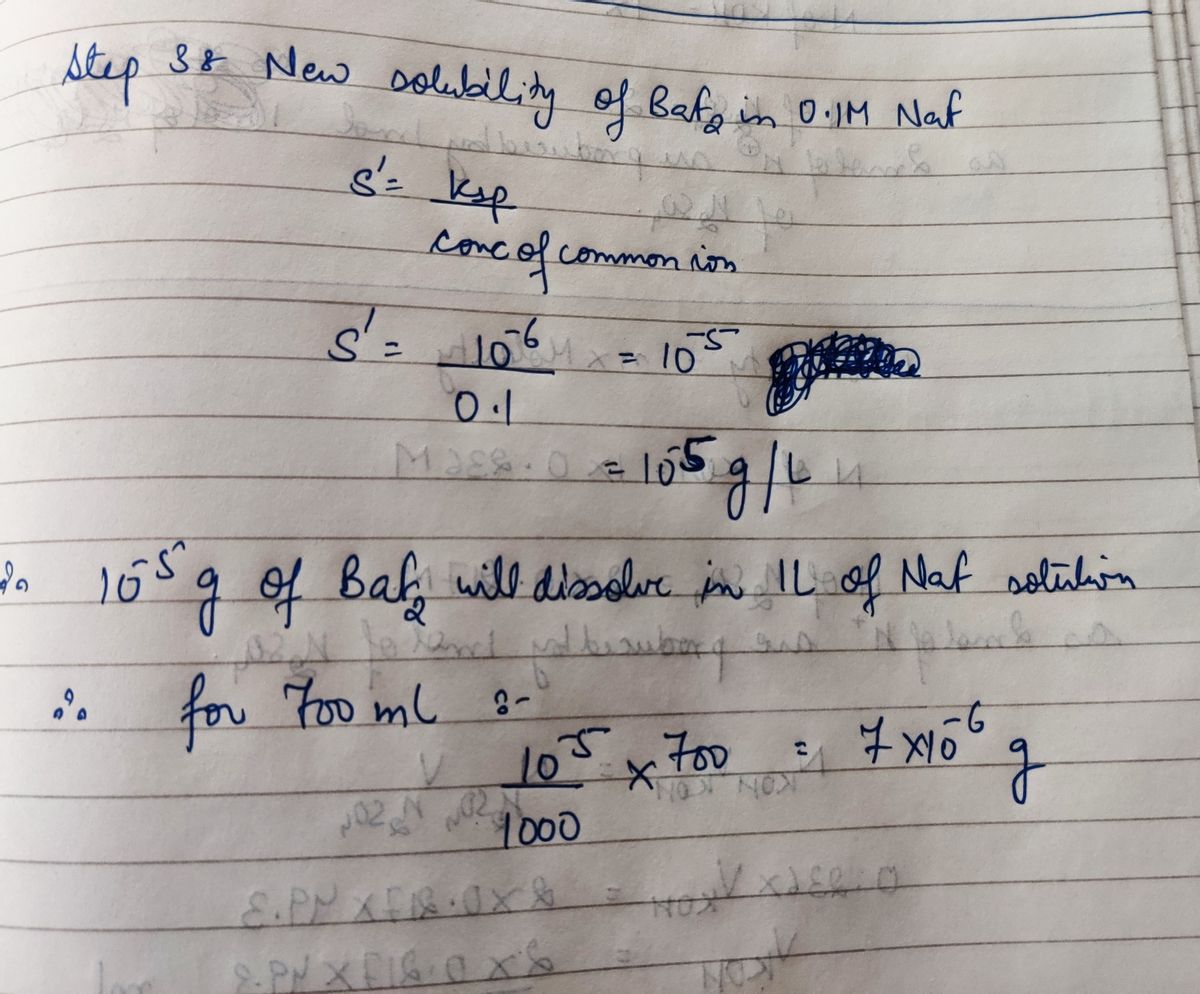
Answered: How many grams of BaF2 (molar mass =…
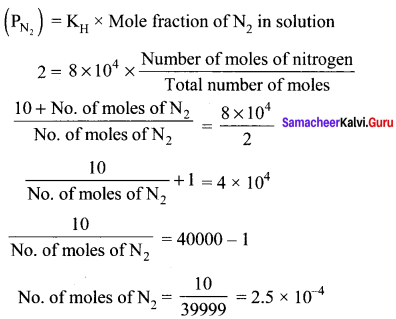
Samacheer Kalvi 11th Chemistry Solutions Chapter 9 Solutions – Samacheer Kalvi
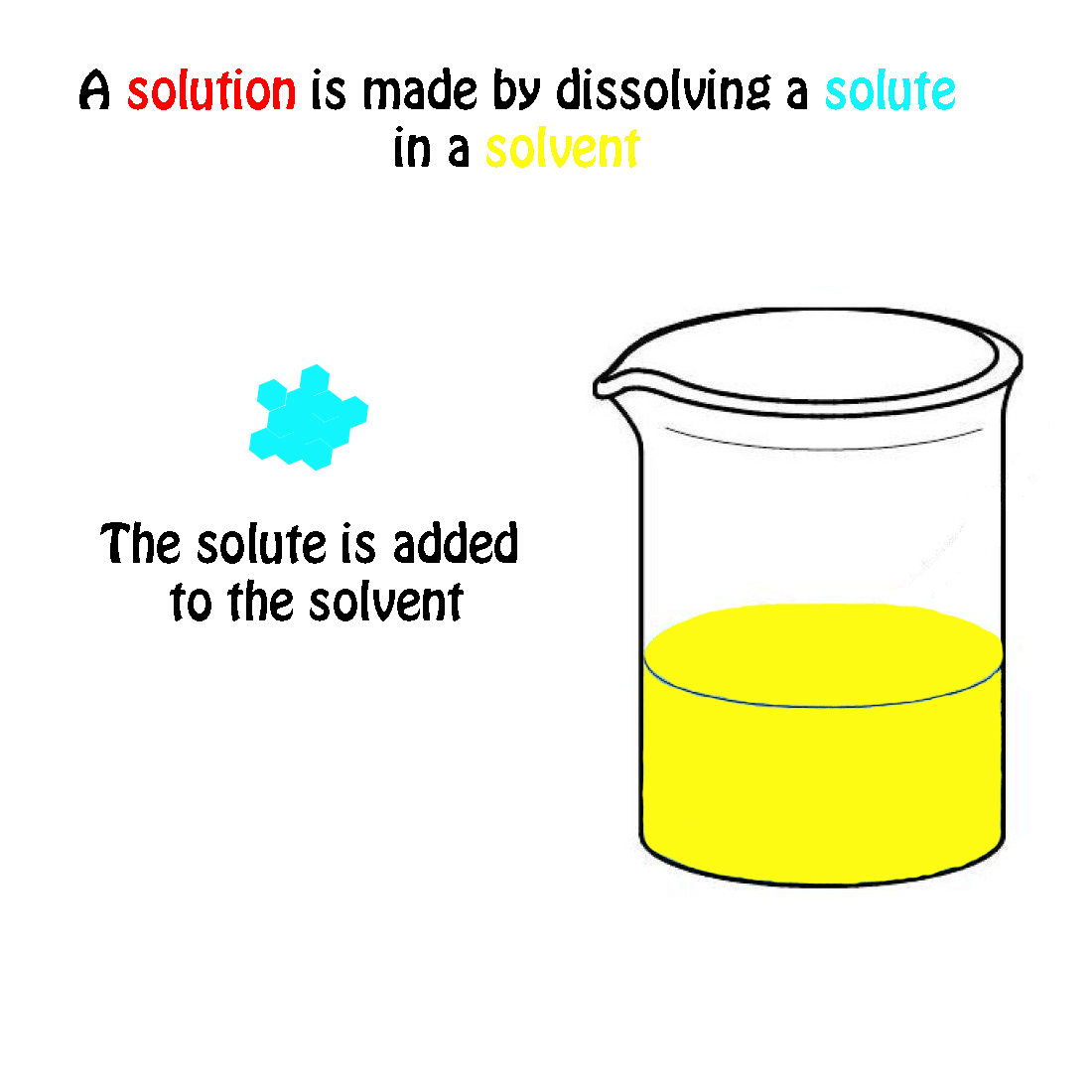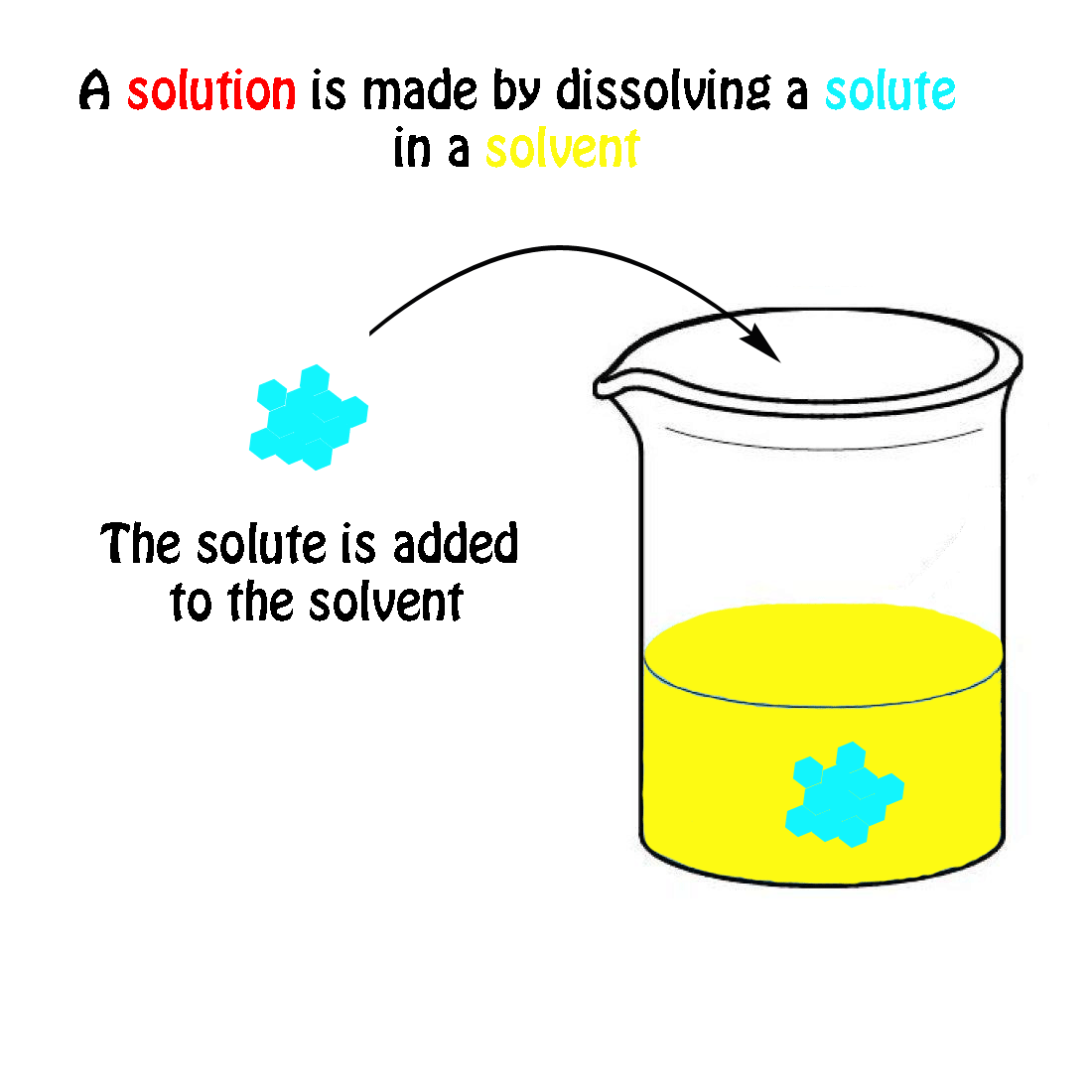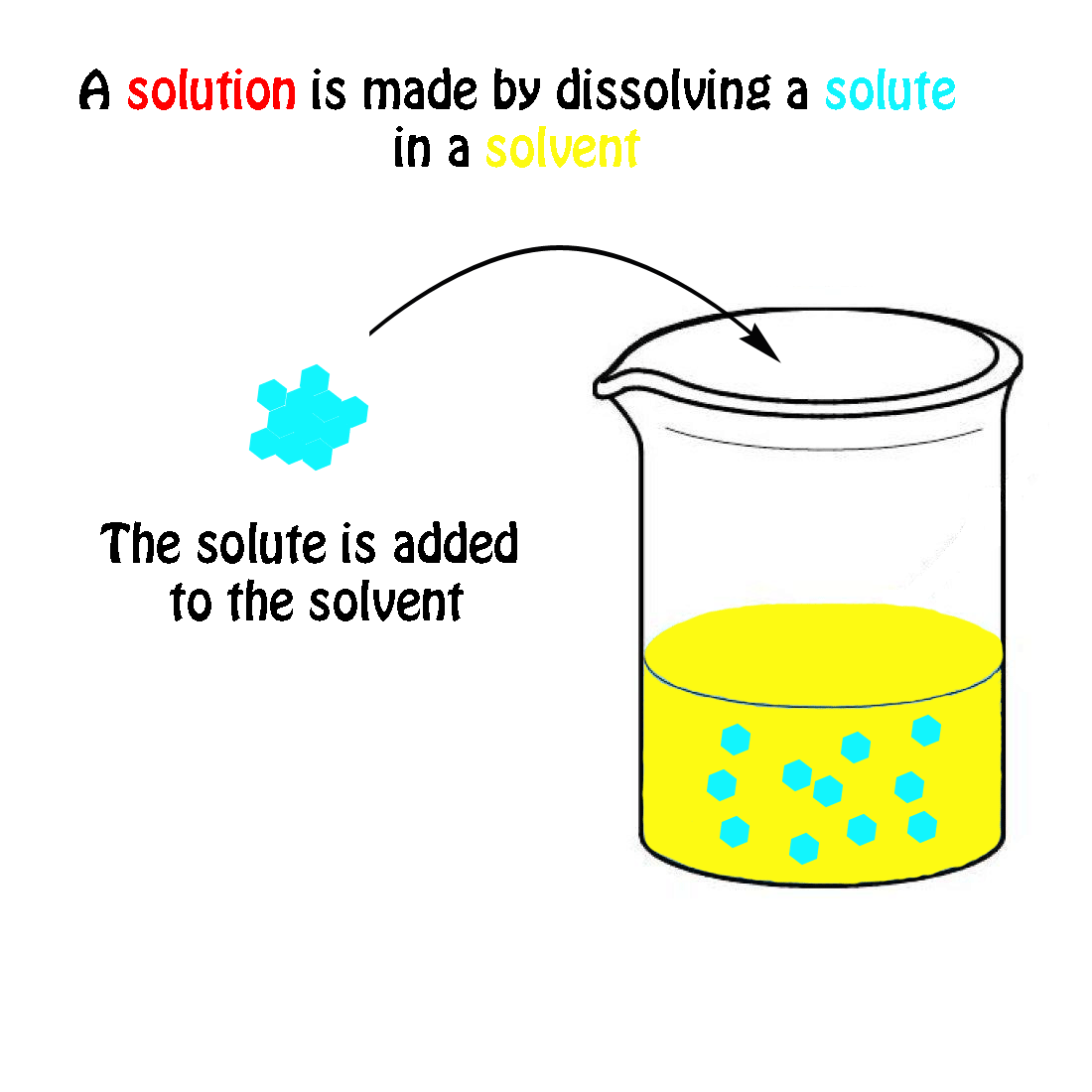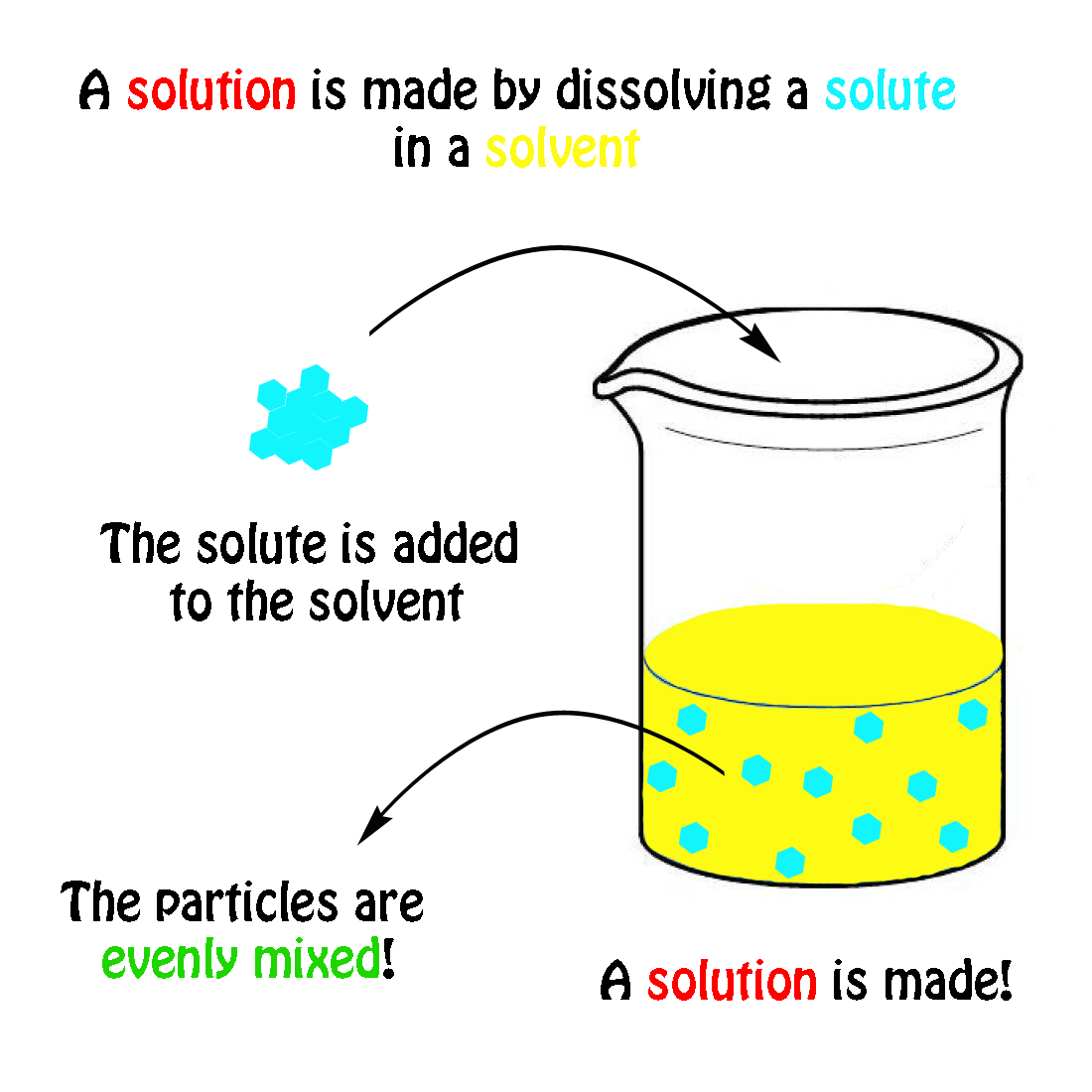
The molecular weight of NaCl is 58.44 grams/mole. If you had a 1.0 molar solution (1.0 M), you would have to put 58.44 g of salt in 1.0 liter of solution. How
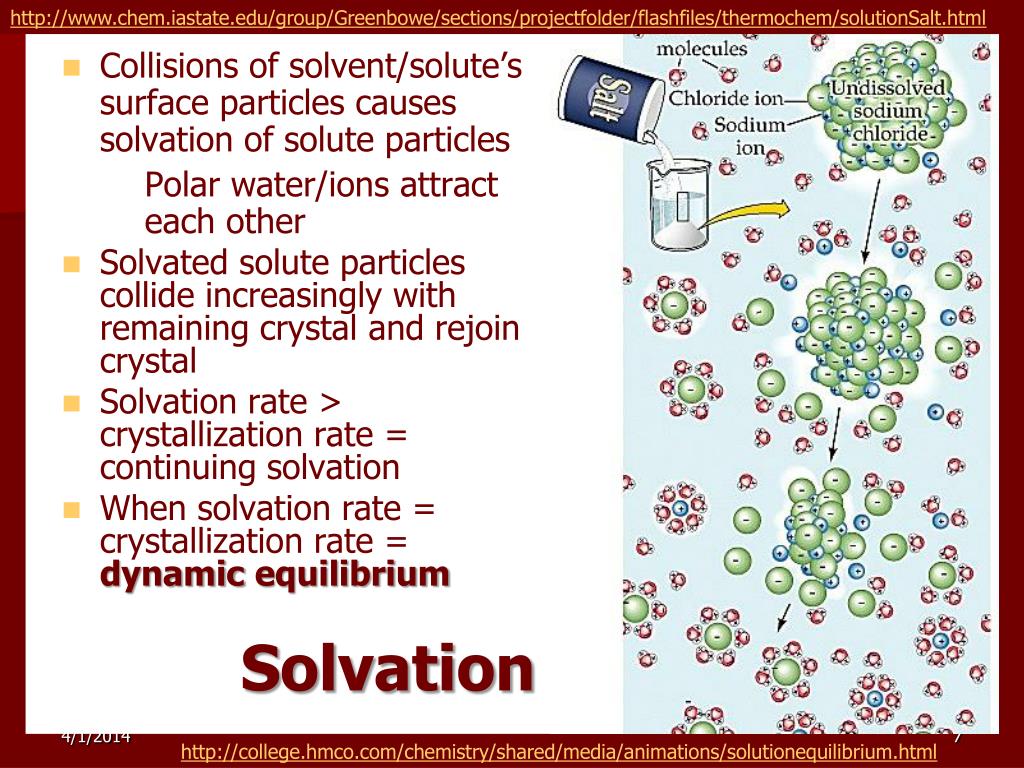
PPT - Solutions PowerPoint Presentation, free download - ID:528392

Lecture Topics Atomic weight, Mole, Molecular Mass, Derivation of Formulas, Percent Composition - PDF Free Download









