CTL Amedica granted FDA 510(k) approval for Navigation Instrument
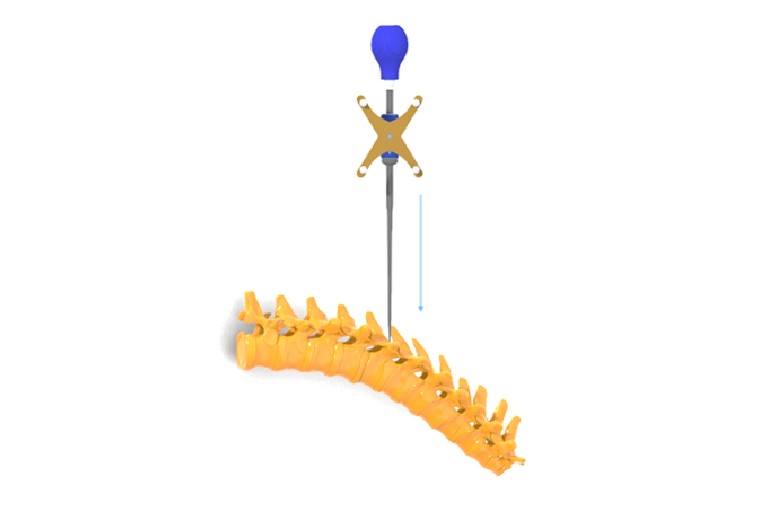

FDA grants 510k clearance of the CTL Amedica Navigation Instrument

FDA Regulation of Medical Devices and Software/Apps
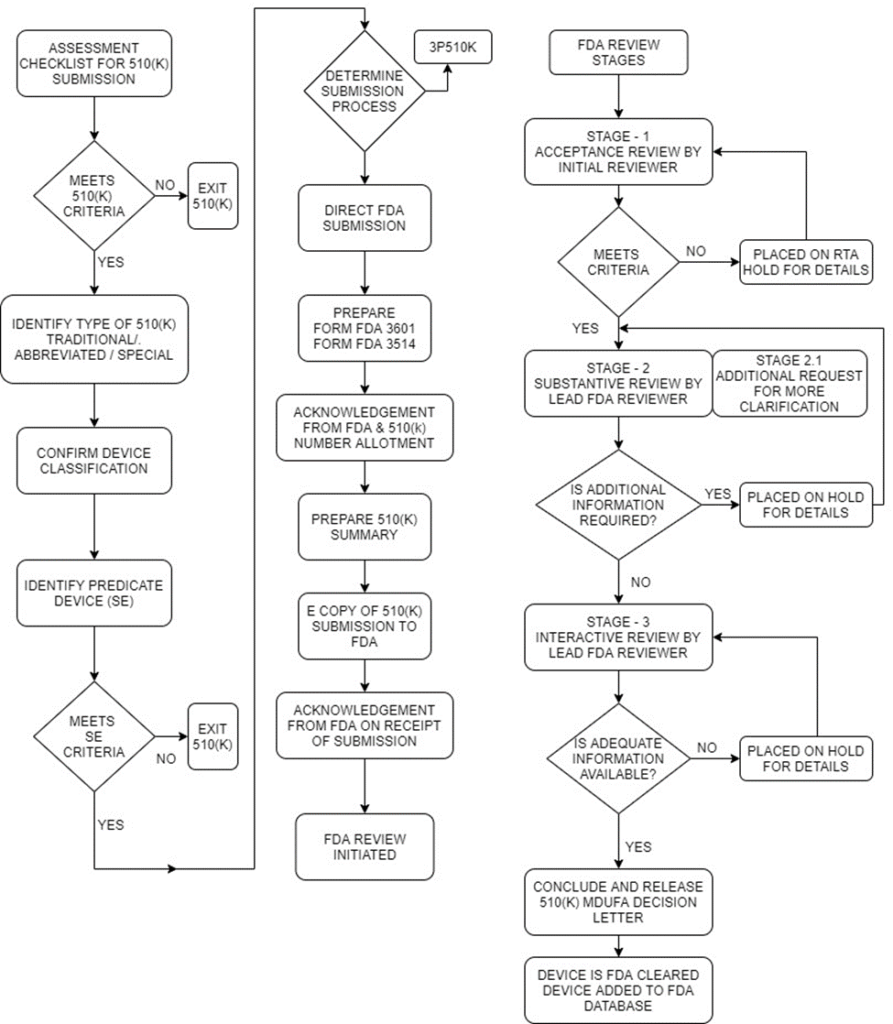
FDA's 510(K) Submission Process

CTL Amedica bags Taiwanese regulatory approval for MATISSE ACIF

Modernizing the FDA's 510(k) Program for Medical Devices

2020 Archives - Page 6 of 16 - SPINEMarketGroup

4 The 510(k) Clearance Process Medical Devices and the Public's
.png)
5 Tips to Help Your FDA 510(k) Submission (checklist included)

2022 Archives - SPINEMarketGroup
_%20Substantial%20Equivalence%20Through%20Performance%20Criteria.png)
Abbreviated 510(k): Substantial Equivalence Through Performance

Darmiyan Receives FDA Approval for BrainSee to Test for

PDF) A Review on Substantial Equivalence of Medical Devices- USFDA

News CTL Amedica
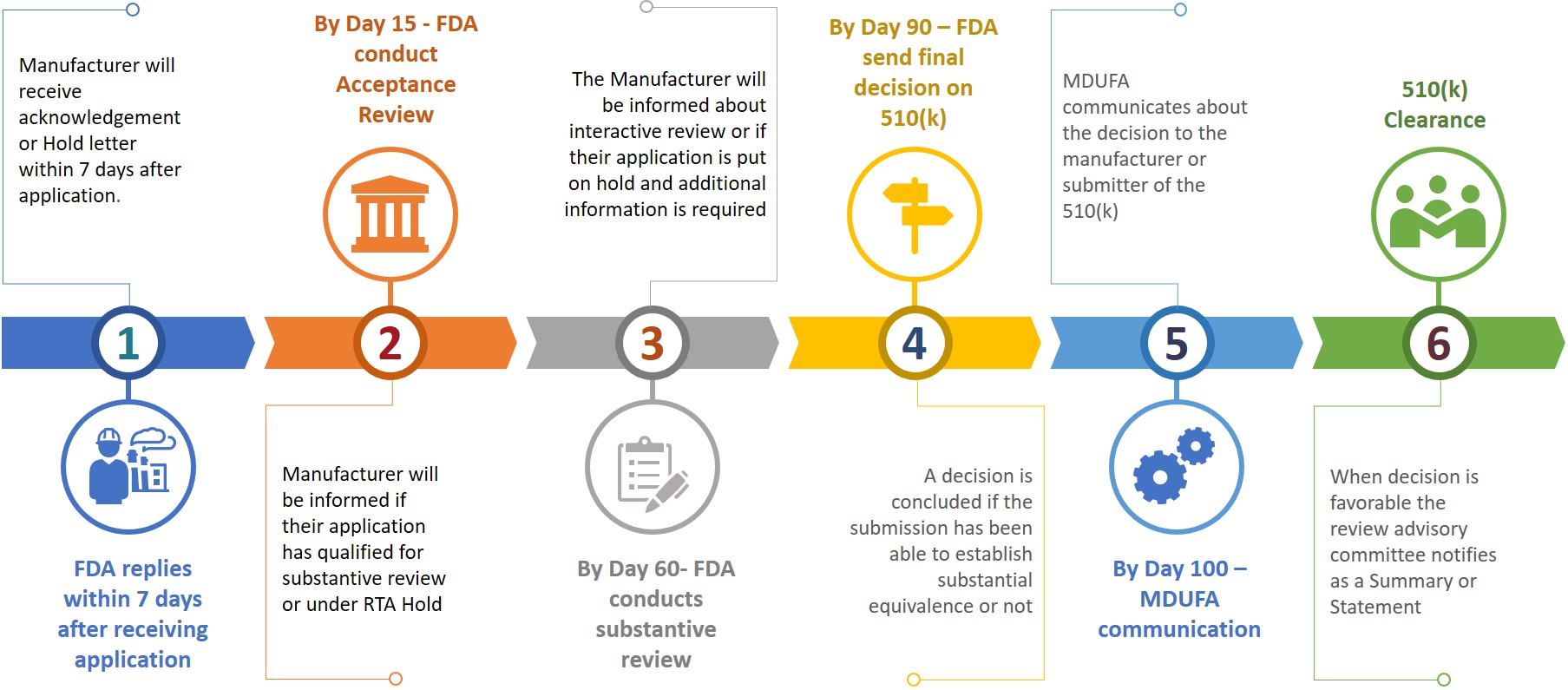
FDA 510k Premarket Notification: Essential Requirements

FDA Guidance on Substantial Equivalence in Premarket Notifications








