Equal heat is gives to two objects A and B of mass 1 g. Temperature of A increases by 3oC and B by 5oC. Which object has specific heat? And by what

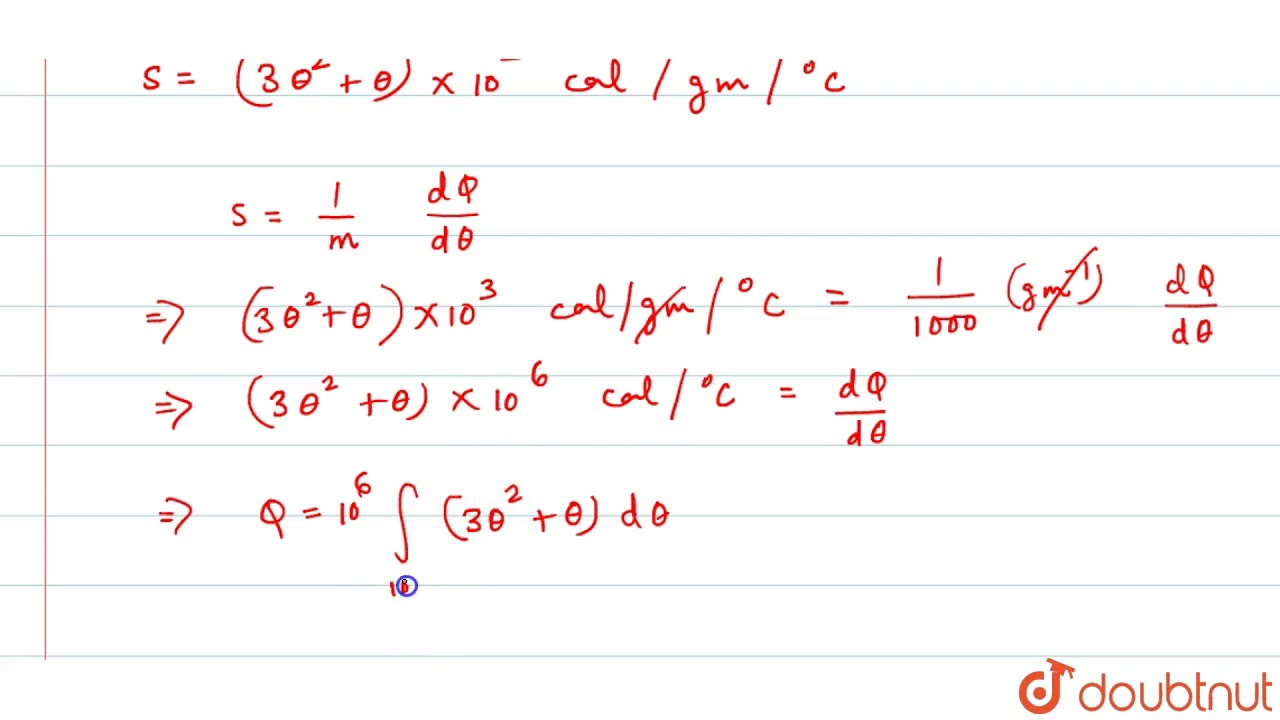
The specific heat of a substance varies as `(3 theta^(2) + theta) xx 10^(3) cal g^(-1) C^(-1)`.
Temperature Change and Heat Capacity

Solar Sensitivity – Watts Up With That?

Specific Heat Capacity (比熱容量) - ppt download

a. Equal heat is given to two objects A and B of mass 1 g. Temperature of A increases by 3 °C and B by 5 Which object has specific heat? And
:max_bytes(150000):strip_icc()/GettyImages-128156856-5ba8f7b44cedfd0025c6835e.jpg)
Find a Reaction's Final Temperature With Specific Heat

Equal heat is given to two objects A and B of mass 1 g. Temperature ofA increases by 3 °C and B by 5 °C.

SOLVED: Equal heat is given to two objects A and B of mass 1 g. Temperature of A increases by 3 oC and B by 5 oC. Which object has more specific

9. Solve the following problems: a. Equal heat is given to two objects A and B of mass 1 g. Temperature of A increases by 3 °C and B by 5 °C.
Engineering.Mechanics.STATICS.Fourteenth.Edition









