Heat of reaction for, CO(g) + 1/2 O2(g)→ CO2(g)at constant V is

Energy Saving and Pollutant Emission Reduction by Adding Hydrogen in a Gasoline-fueled Engine - Aerosol and Air Quality Research
What is the equilibrium constant (k) expression of CO2 = CO + O2? - Quora

Heat of reaction for, CO(g) + 1/2 O2(g)→ CO2(g)at constant V is 67.71 K cal at 17^° C. The heat of reaction at constant P at 17^° C is

Heat of reaction . CO(g) + 1/O2(g) → CO2(g) constant V is-67.71 K 17℃ The heat of reaction constant P 17°C is (1-68 K a 2. 1S- (91

Ch.16
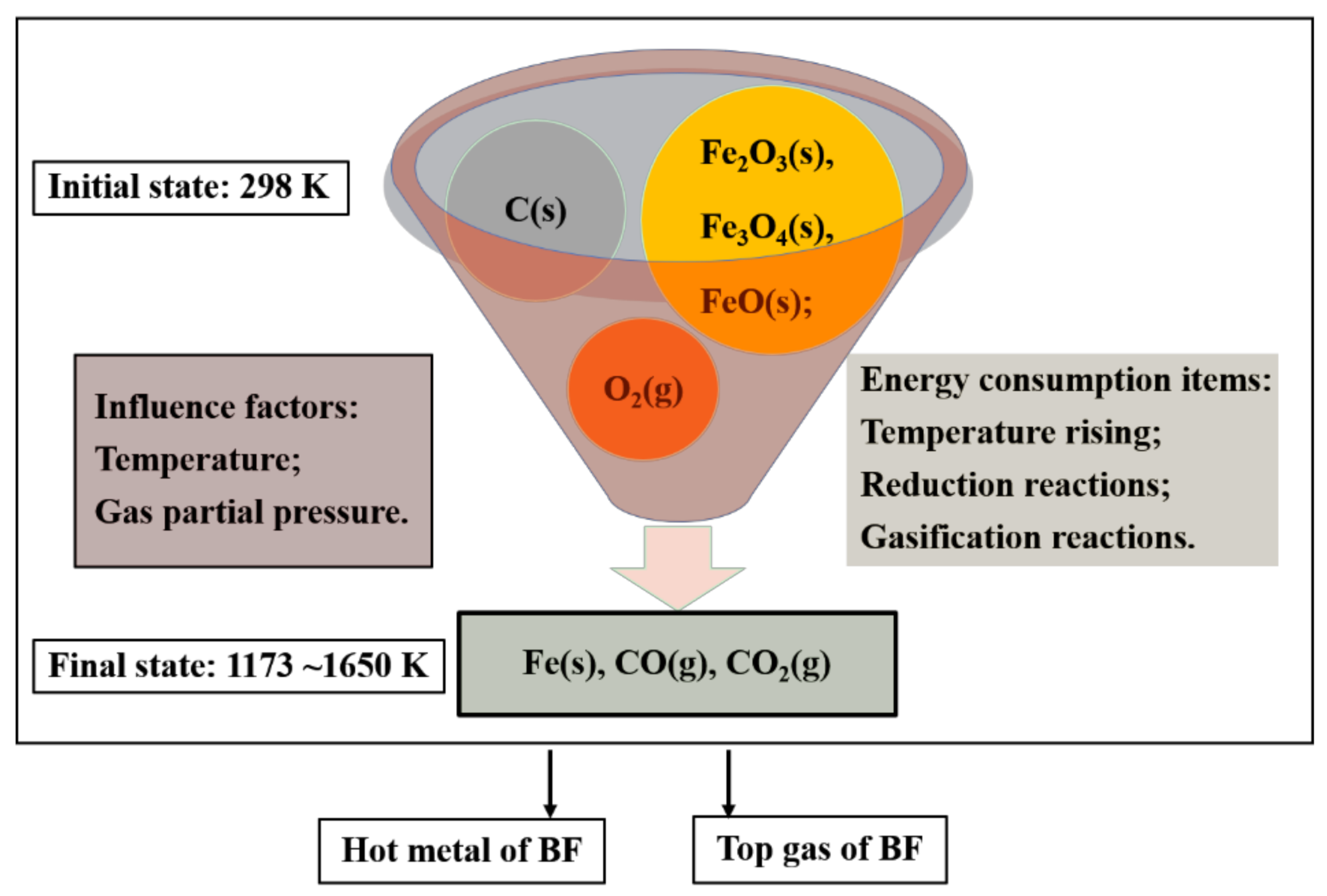
Energies, Free Full-Text
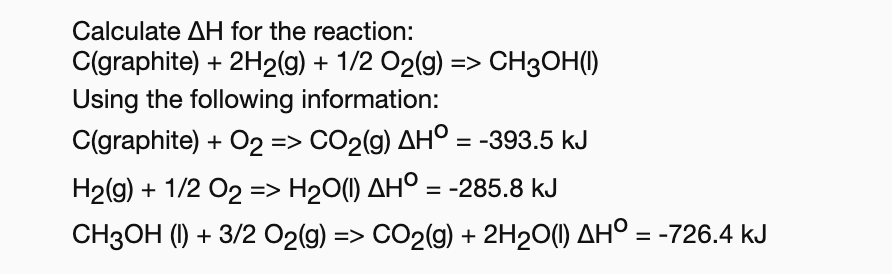
Answered: Calculate AH for the reaction:…

The enthalpy of combustion of solid carbon to form CO2 is -393.7 kJ/mol
What is the rate law equation NO2 + CO -------------NO + CO2? - Quora

exam questions I got wrong Flashcards
Heat of reaction for, CO(g) + 1/2 O2(g)→ CO2(g)at constant V is 67.71 K cal at 17^° C. The heat of reaction at constant P at 17^° C is

20 dm?, AH is - (3) 3.46 kJ (2)-1.73 KJ (1) 1.73 KJ Heat of reaction CO(g) + 12 O. (g) → CO, (g) constant V is -67.71 Kcal 17°C. Thebe constant P 17°C is :- -2.6277 (4) None (3) -67.42 K (2) + 68.0 Kcal (1) -68.0 kcal

Solved Question 27 (1 point) In the reaction, CO (g) + NO2
Carbon Dioxide, CO2

5.68a Calculate the standard enthalpy change for N2(g) + O2(g) → 2NO(g)









